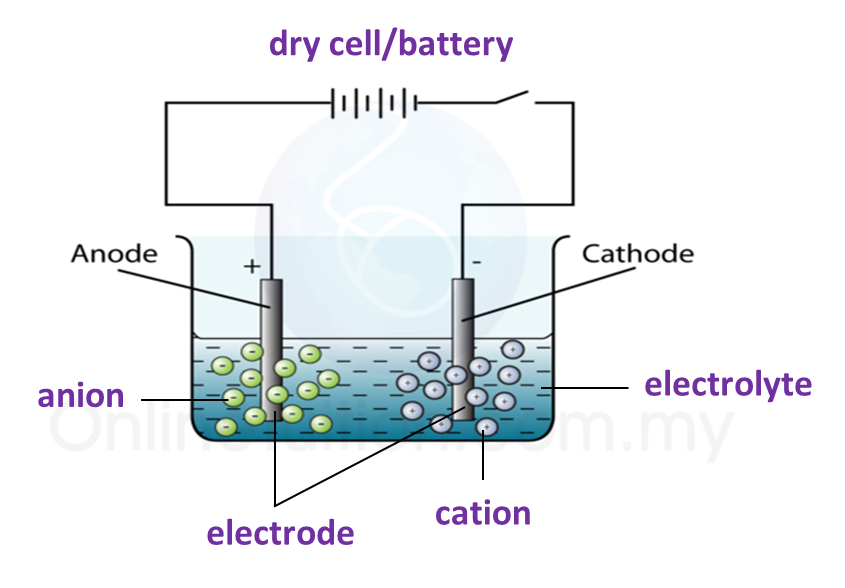
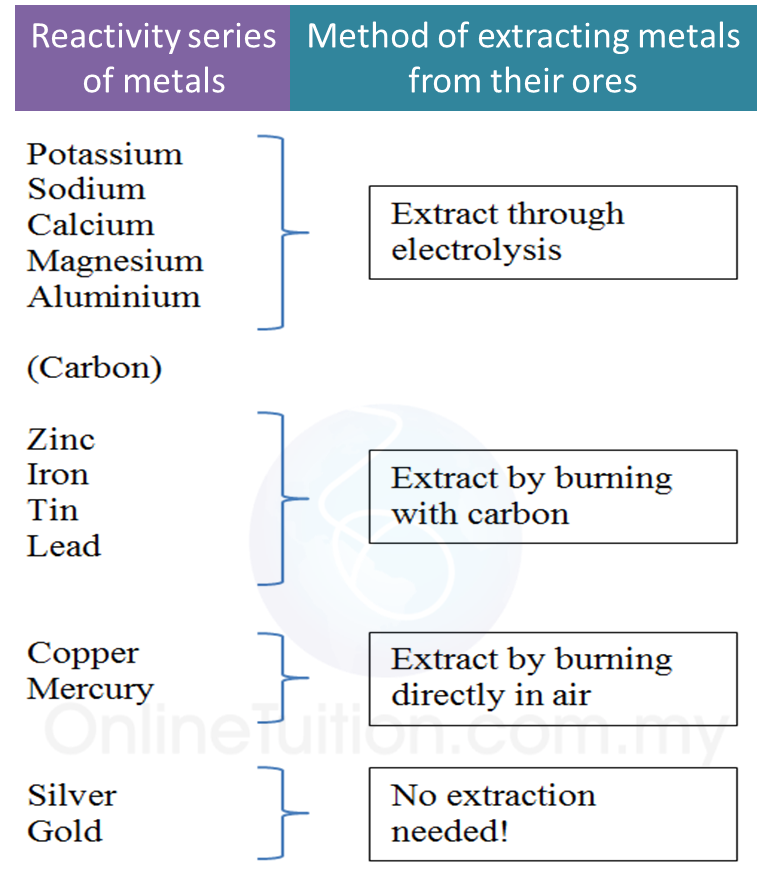
5.4.3 Extraction of Metals from their Ores Using Coke
1. In industries, metal ores which are less reactive than carbon are heated with carbon to obtain pure metal.
2. Pure metals which can be extracted using carbon are zinc, iron, tin and lead.
Extraction of Tin from its Ore
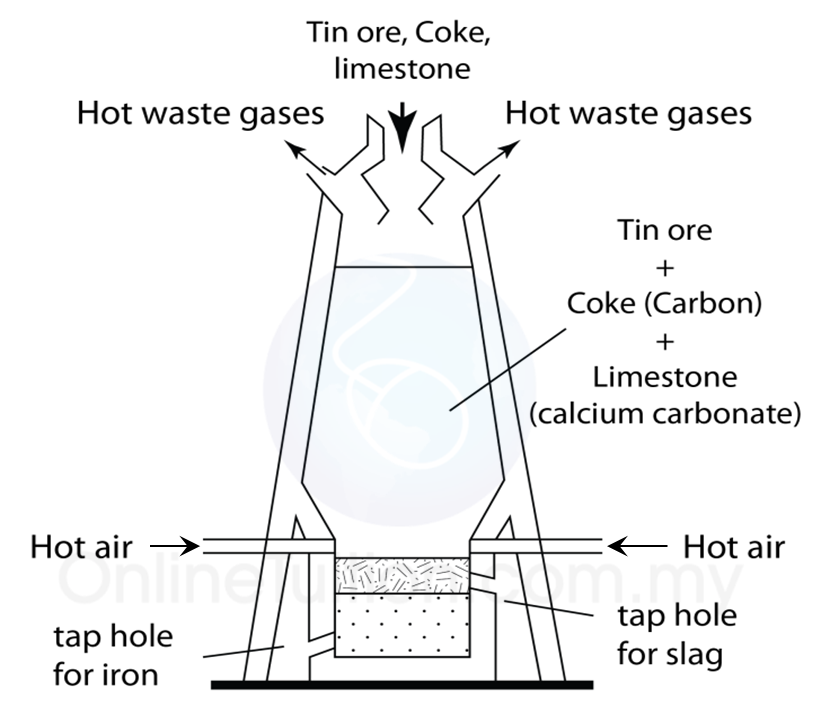
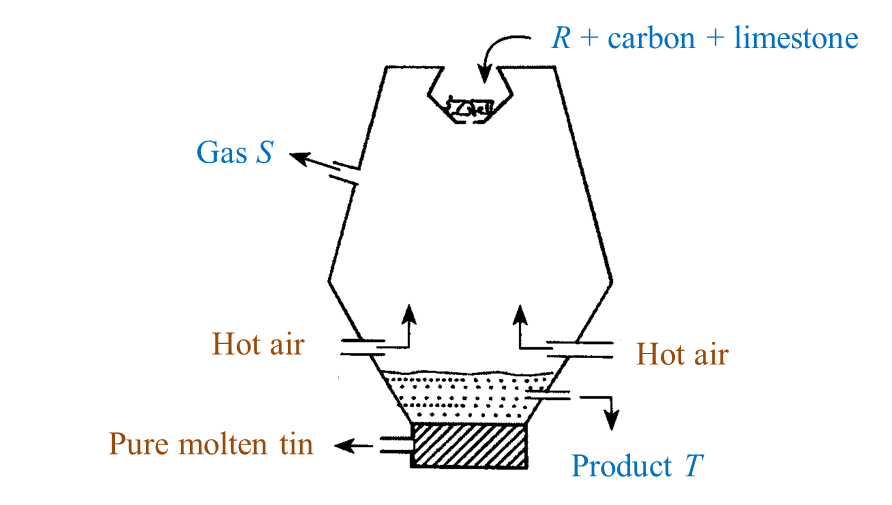
Extracting tin ore in a blast furnace
1. Tin ore exists naturally as cassiterite (or tin oxide).
2. Tin ore is washed with water to remove sand, clay and others impurities.
3. After that, tin ore is roasted to take away foreign matter such as carbon, sulphur and oil.
4. Lastly, the tin ore is mixed with carbon and limestone in the form of coal and is heated in a blast furnace at a high temperature.
5. The function of the limestone is to remove impurities.
6. Reduction reaction occurs during heating, carbon which is more reactive that tin removes oxygen from the tin oxide to produce pure tin and carbon dioxide.

7. Pure tin flows out from the furnace into moulds to harden as tin ingots.
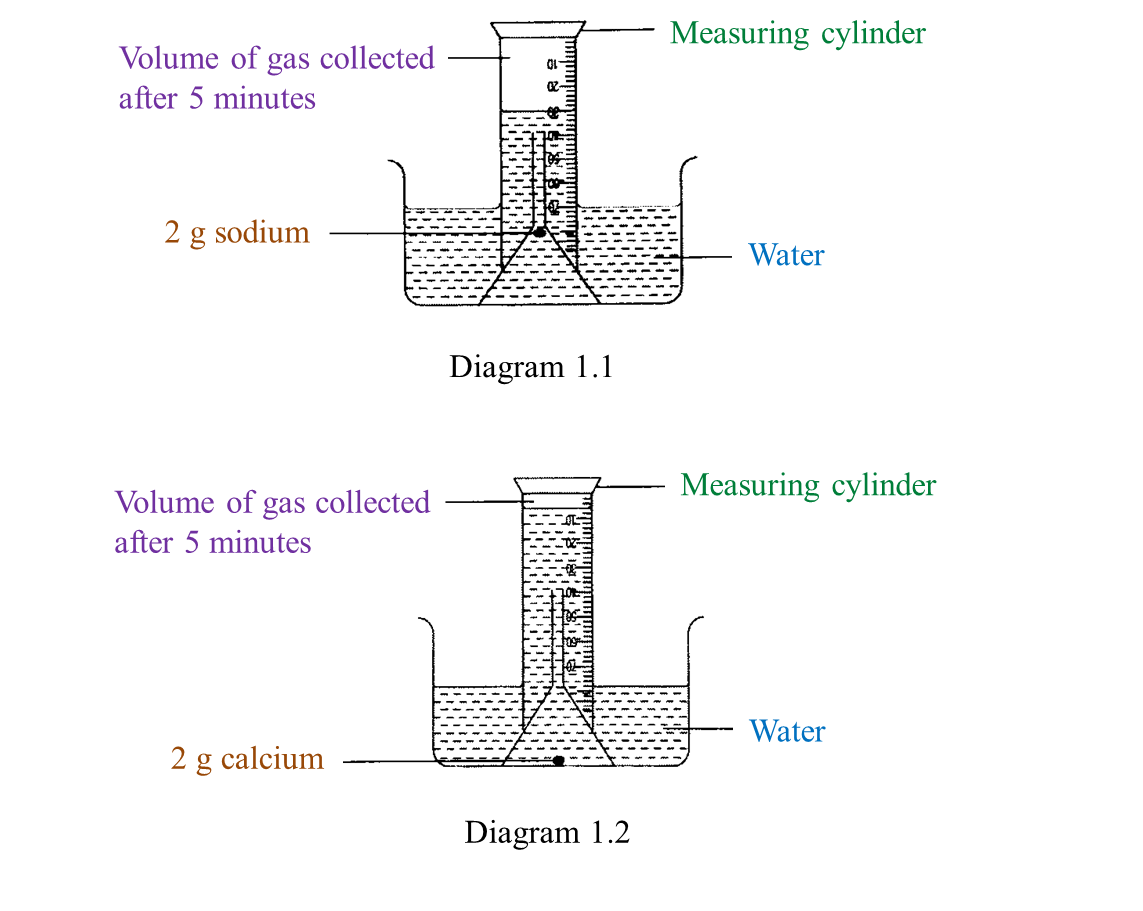
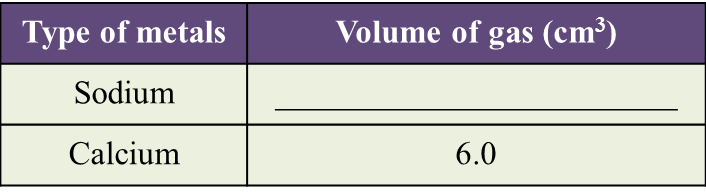
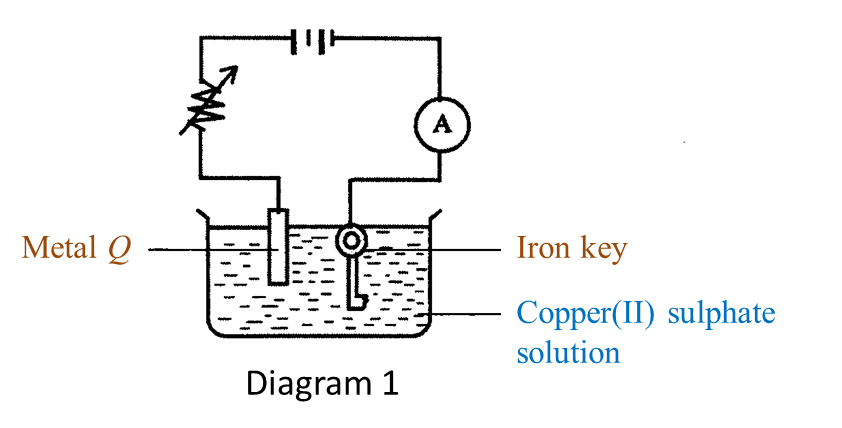 (a) Name the process in Diagram 1. [1 mark]
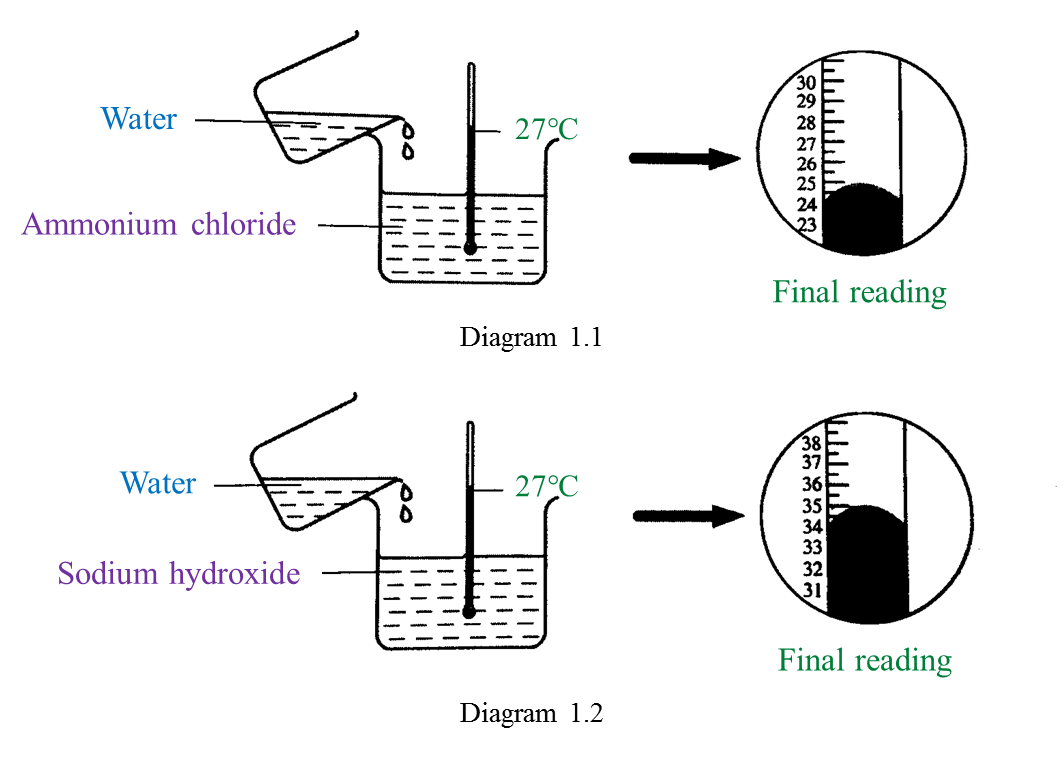
(a) Name the process in Diagram 1. [1 mark]