[adinserter block="3"]
[adinserter block="3"]
[adinserter block="3"]
Transport of Nutrients by Circulatory System
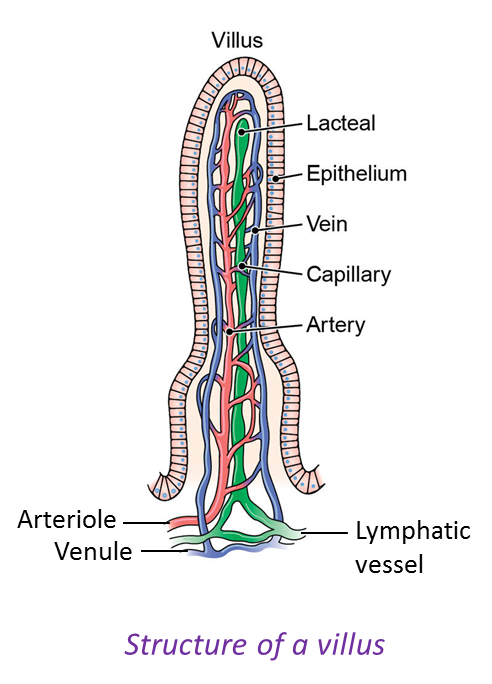
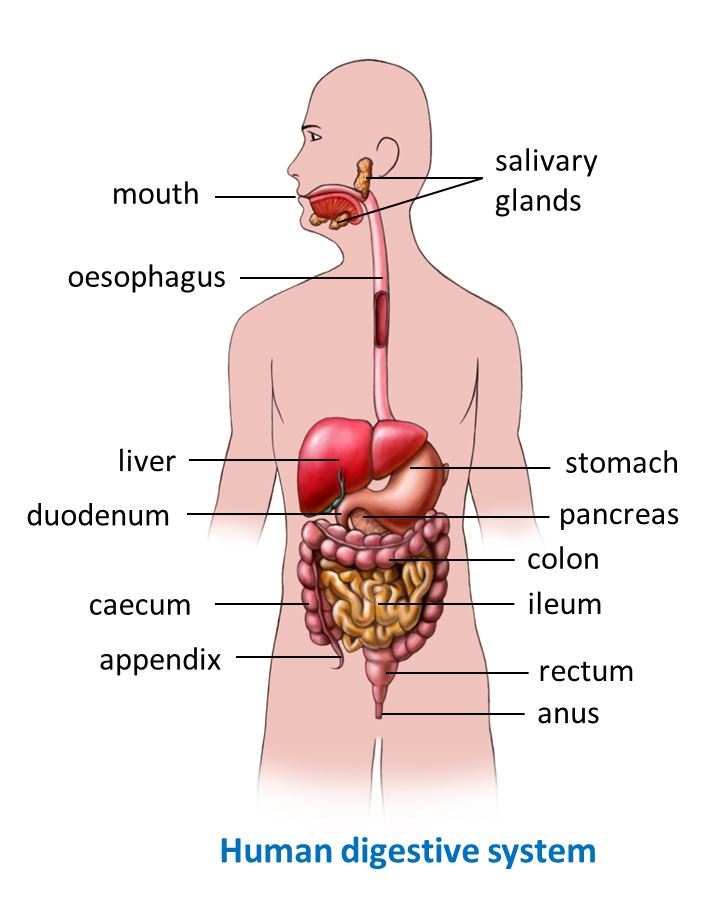
1. Glucose, amino acids, minerals, vitamins B and C are absorbed into blood capillaries of the villus and carried out of the small intestine to the liver by the hepatic portal vein.
2. The hepatic portal vein transports the nutrients absorbed from the small intestine to the liver for processing and prepared for metabolic process or assimilation.
3. Assimilation takes place in the cells where the nutrients are used to form complex compounds or structural components, for example, the transformation of amino acids to proteins.
4. Glycerol and fatty acids as well as vitamins A, D, E and K are absorbed into the lacteal of the villus and transported out of the small intestine by the thoracic duct to the lymphatic system. The lymph is then transported by the thoracic duct to the subclavian vein. The lipid droplets and fat-soluble vitamins are then transported to all the cells via the blood circulatory system.
[adinserter block="3"]
Functions of the Liver
1. Regulation of blood glucose levels
Convert excess glucose to glycogen for storage, and glycogen to glucose when the glucose level is low.
2. Storage of nutrients
(a) Excess glucose is converted into fats in the liver and later stored in other parts of the body.
(b) The liver also stores iron and fat soluble vitamins such as A, D, E and K.
3. Deamination
Breaks down excess amino acid to ammonia in a process called deamination. Ammonia is then converted to urea and is excreted in the urine.
4. Detoxification
The liver detoxifies blood by removing and metabolizing toxic substances.
5. Production of bile
(a) Bile contains bile salts and bile pigments which are delivered to the duodenum.
(b) Bile emulsifies lipid droplets into tiny droplets. This increases the surface area of the action for the lipase.
6. Synthesis of plasma proteins
The liver is the site of plasma protein synthesis, for example, fibrinogen and prothrombin, which are vital blood clotting agents, are synthesized in the liver.
[adinserter block="3"]
Assimilation
1. Assimilation is the process by which food materials after being absorbed are built up into complex constituents of the organism.
2. Glucose
· Glucose is oxidised in body cells during respiration to produce energy for various
activities such as cell division.
· Excess glucose is converted into glycogen and stored in the liver.
· When the blood sugar level falls and the body needs energy, the stored glycogen is converted back into glucose.
3. Amino Acids
· Amino acids are absorbed by body cells to synthesise various types of proteins such as enzymes, antibody, plasma membrane and protoplasm.
· Excess amino acids are broken down in the liver and converted into urea which is excreted in the urine through the process of deamination.
4. Lipids
· Lipids (fat droplets) are absorbed by body cells to build the plasma membrane and cholesterol.
· Phospholipids are components of the plasma membrane.
· Excess fat are stored in adipose tissue underneath the skin as reserve energy and around internal organ.
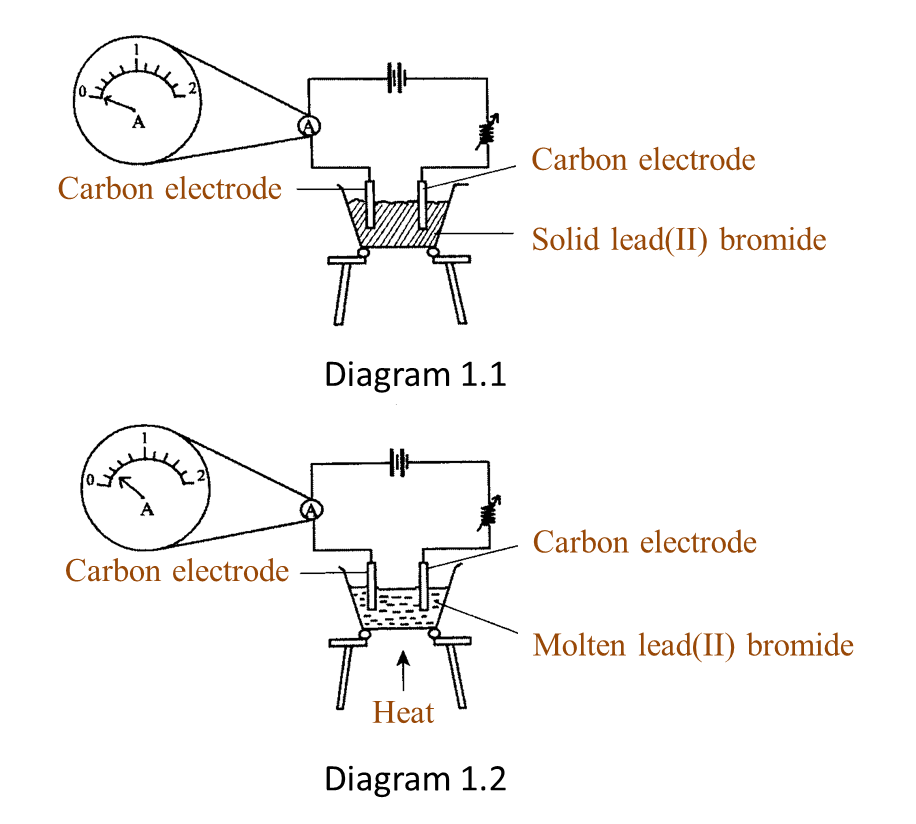
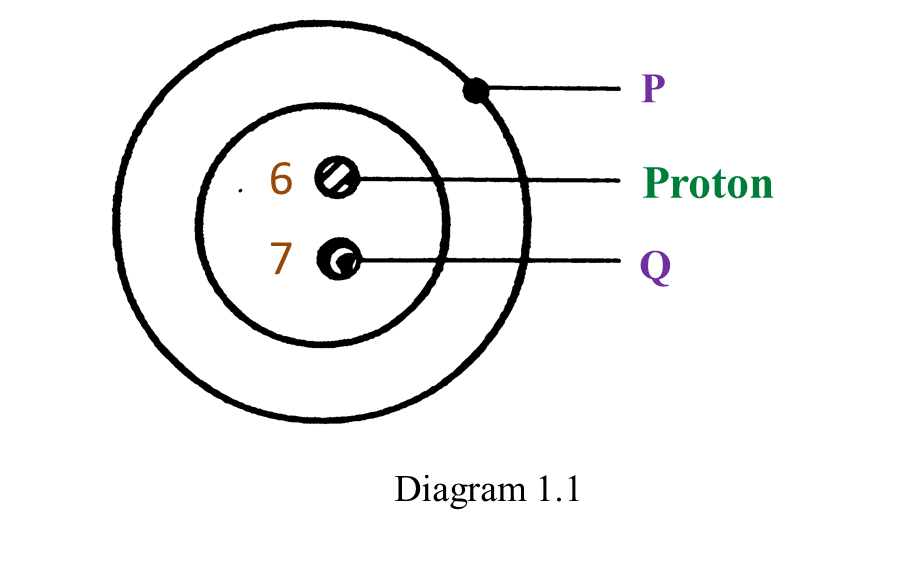 (a) P and Q are subatomic particles.
(a) P and Q are subatomic particles.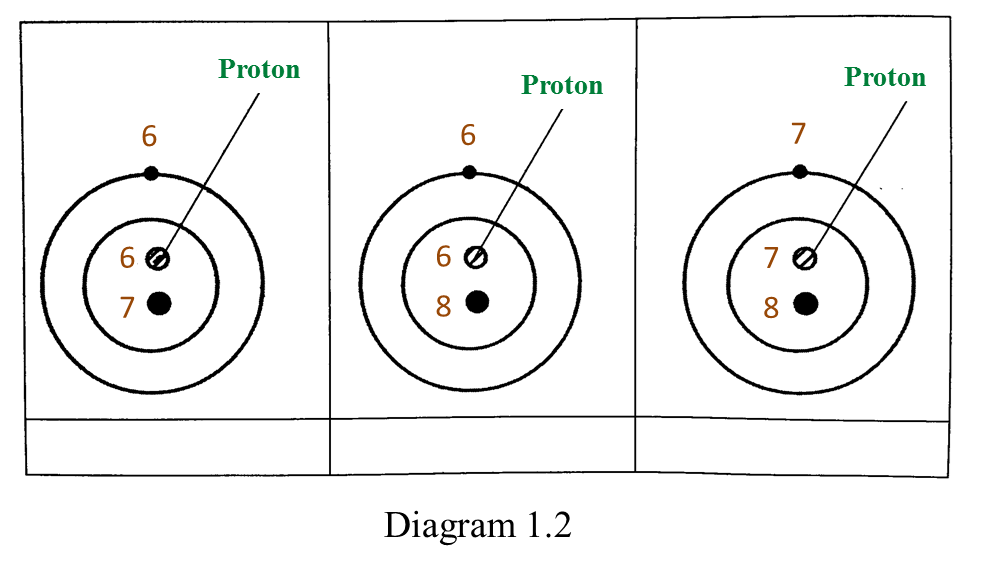
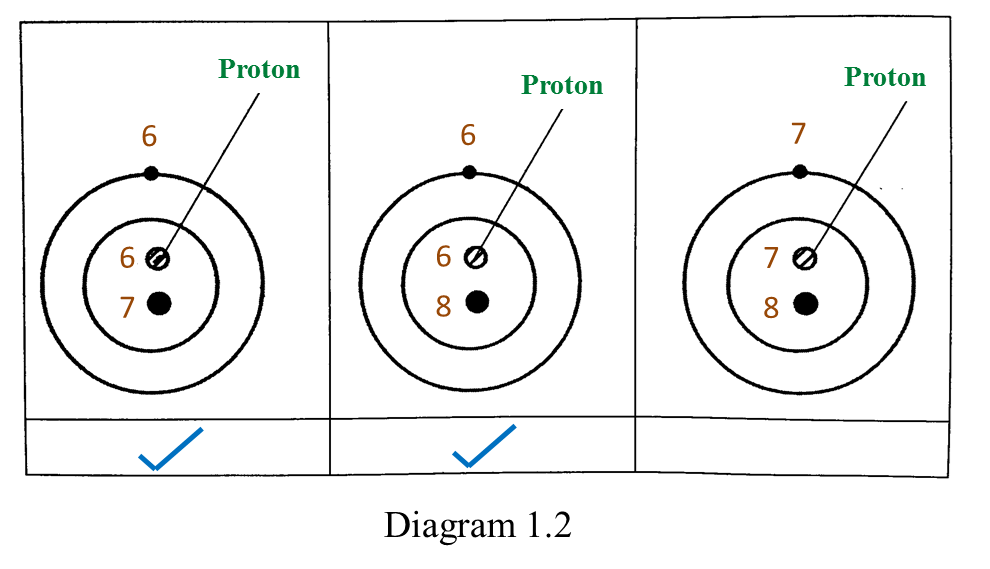
 Based on the Diagram,
Based on the Diagram,