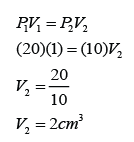Boyle's law states that the pressure of a gas with constant mass is inversely proportional to its volume provided the temperature of the gas is kept constant.
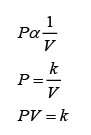
Formula:
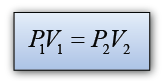
Explanation
- When the volume of gas decreases, the number of gas particles per unit volume increases.
- As a result, the frequency of collision between the air particles and the wall of the container increases.
- As such, the pressure of the gas increases.
Graph
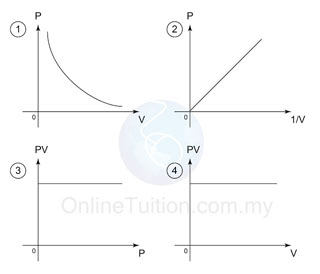
- In the graphs above, the first graph shows that P is inversely proportional to V.
- The second graph shows that P is directly proportional to 1/V.
- The third and the forth graphs shows that PV is always constant for all value of V and P.
Example 1:
A fish releases a bubble of air of volume 1cm³ at the bottom of a lake. The depth of the lake is 10m. Find the volume of the bubble when it reaches the surface of the pond. (Assume that the atmospheric pressure is equal to 10m of water)
Answer:
V1 = 1cm³
P1 = 20m water
V2 = ?
P2 = 10m water