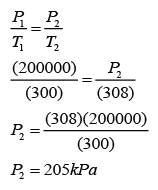Pressure law states that for a fixed mass of gas, the pressure of the gas is directly proportional to the absolute temperature of the gas provided the volume of the gas is kept constant.
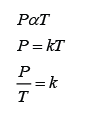
Formula:
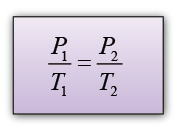
Explanation
- The kinetic energy of gas molecules increases with temperature.
- The air molecules collide with the wall of the container at higher velocity and frequency.
- The pressure in the gas increases, causing an increase in volume.
Graph
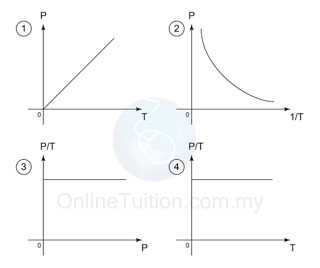
- In the graphs above, the first graph shows that P is directly proportional to the absolute temperature.
- The second graph shows that, if the temperature is in °C, the graph does not pass through the origin.
- The third and the forth graphs shows that P/T is always constant for all value of P and T.
Example 2:
An iron cylinder containing gas with pressure 200kPa when it is kept is a room of temperature 27°C. What is the pressure of the gas when the cylinder is located outdoor where the temperature is 35°C.
Answer:
P1 = 200kPa
T1 = 273 + 27 = 300K
P2 = ?
T2 = 273 + 35 = 308K