Figure below shows the change of the temperature when a solid is heated until it become gas.
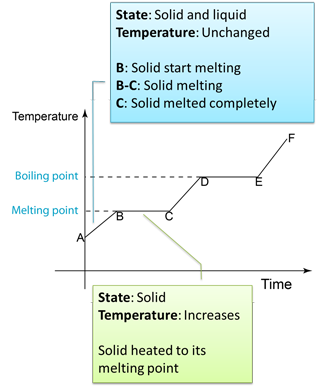
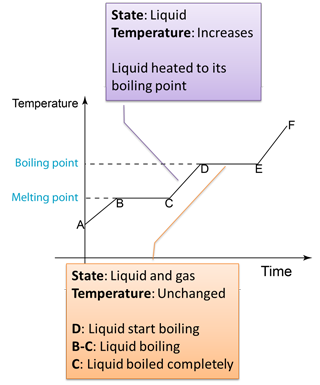
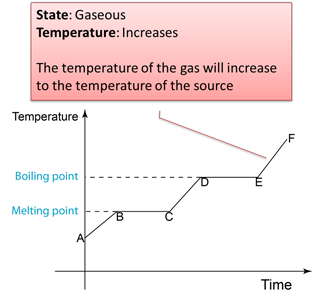
Note:
B to C
- Temperature remain unchanged because the heat absorbed is used to overcome the force between the particles in the solid.
- The heat absorbed to change a solid to liquid is called the latent heat of fusion.
D to E
- Temperature remain unchanged because the heat absorbed is used to overcome the force between the particles in the liquid and also the atmospheric pressure.
- The heat absorbed to change a liquid to gas is called the latent heat of vaporisation.