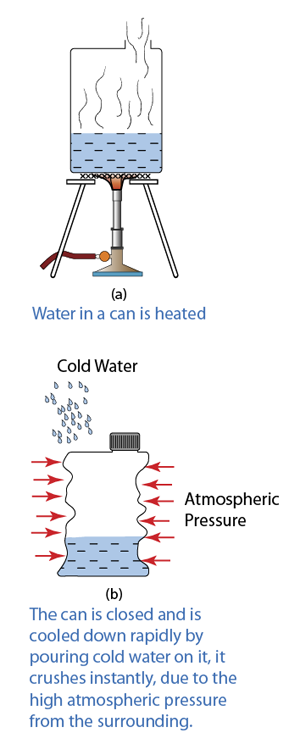
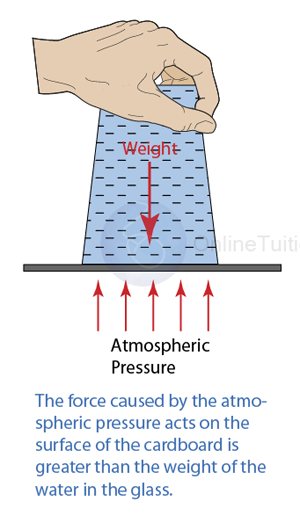
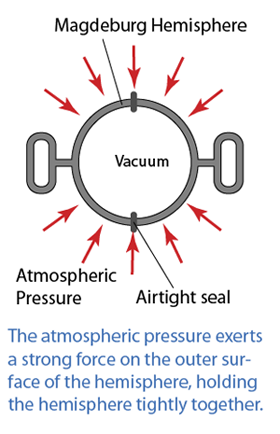
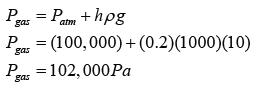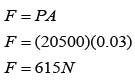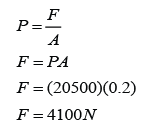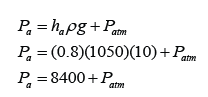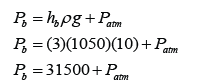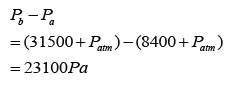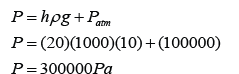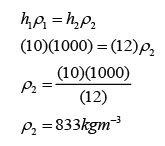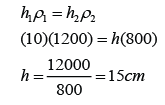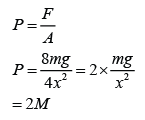Instruments Used to Measure Gas Pressure
- The pressure of the gas in a container can be measured by using
- Bourdon gauge
- Manometer
- In SPM, almost all calculation question about using instruments to find gas pressure in a container are related to manometer. Therefore it's important for you to know the concept behind this instrument.
- For the Bourdon Gauge, you need to know its working mechanism.
Bourdon Gauge
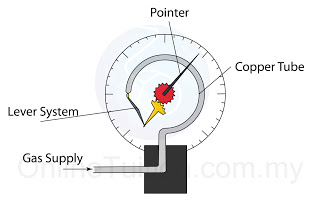
- A Bourdon gauge is used to measure to gas pressure in a container.
- There are 3 important components in a Bourdon gauge
- the copper tube
- the lever system
- the pointer
Working Mechanism of a Bourdon Gauge
- When the gauge is connected to a gas supply, the gas pressure will straighten the copper tube.
- The copper tube exerts a force on the lever system and hence move the pointer. The movement of the copper tube is magnified by the lever system
- The pointer rotates and give a reading (in unit of Pascal).
Manometer
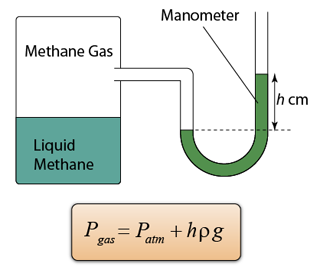
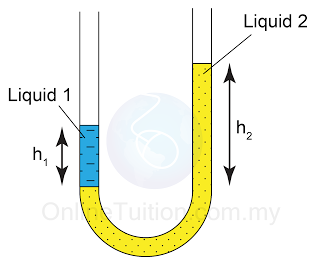
- A manometer is a U-shape tube filled with some liquid (usually mercury).
- Manometer is a device used to measure gas pressure in a container.
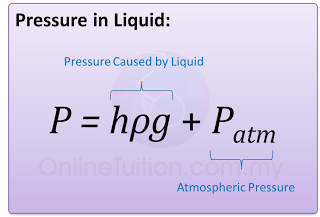
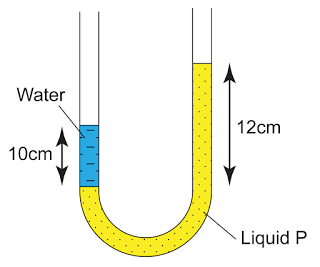
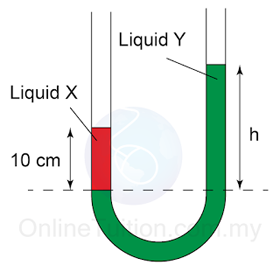
- The pressure of the gas is equal to the sum of the atmospheric pressure and pressure due to the column of liquid.
Note:
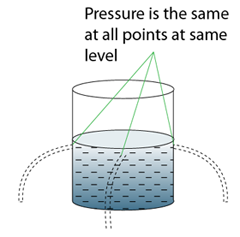
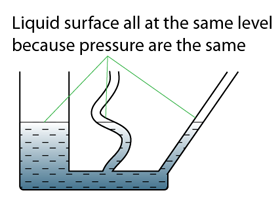
There are a few points we need to know when using a manometer- Difference in gas pressure at difference level can be ignored.
- Pressure on the surface of liquid is equal to the gas pressure in contact.
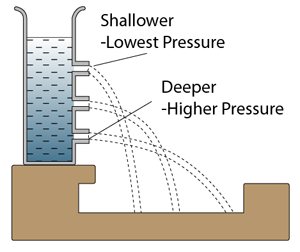
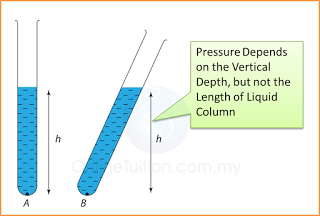
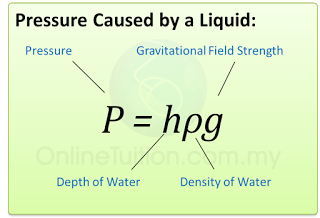
- Pressure that cause by liquid = hρg.
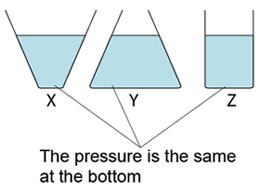
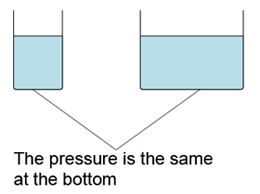
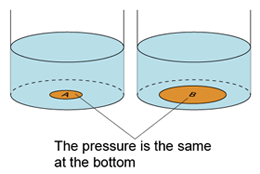
- For a given liquid, the pressures at any point of the same level are the same.
- For different liquid with different density, pressure at two different level will be different.
Example 1:
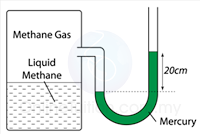
Figure above shows a manometer containing mercury connected to a tank with methane liquid and gas. Find the pressure of the gas supply in the units cmHg and Pa.
[Density of mercury = 13.6 x 10³ kg/m³; atmospheric pressure = 76 cmHg]
Answer:
Pressure of the gas in cmHg
P = 20 + 76 = 96 cmHg
Pressure of gas in Pa
The atmospheric pressure,
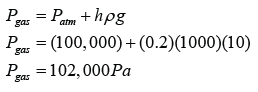
Pressure of the gas,
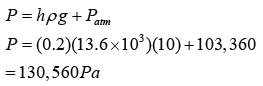
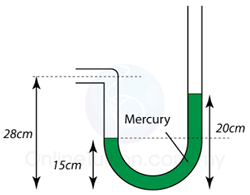
Figure above shows the mercury levels in a manometer used to measure the pressure of a gas supply. How much is the gas pressure greater than the atmospheric?
Answer:
- The pressure of the gas trapped in a capillary tube depends on the position of the tube.
- Figure below shows the pressure of the gas when the capillary tube is horizontal, vertical and vertically upside down.
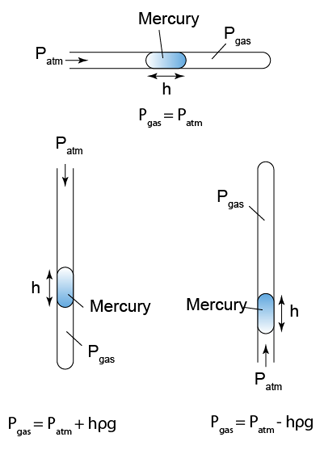
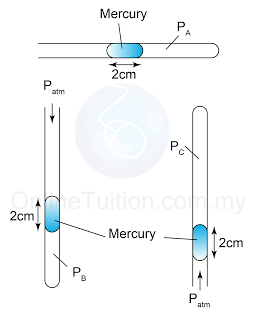
Figure above shows 3 identical capillary tubes with one end sealed and containing a column of mercury. PA, PB and PC are the gas pressure in the capillary tubes respectively. Find the value of PA, PB and PC. [Atmospheric pressure = 76cmHg]
Answer:
PA = 76cmHg
PB = 76cmHg + 2cmHg = 78cmHg
PC = 76cmHg - 2cmHg = 74cmHg
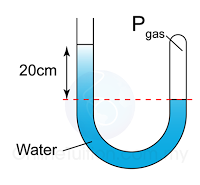
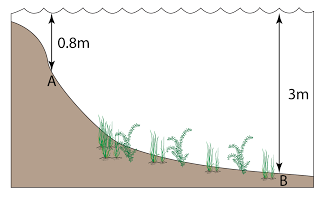
Figure above shows some air trapped in a J-tube. Find the pressure of the trapped air. [Density of water = 1000 kg/m³; Atmospheric pressure = 100,000 Pa]
Answer: