[adinserter block="3"]
Question 1:
(a) Diagram I shows the effects of farming activities near a pond.
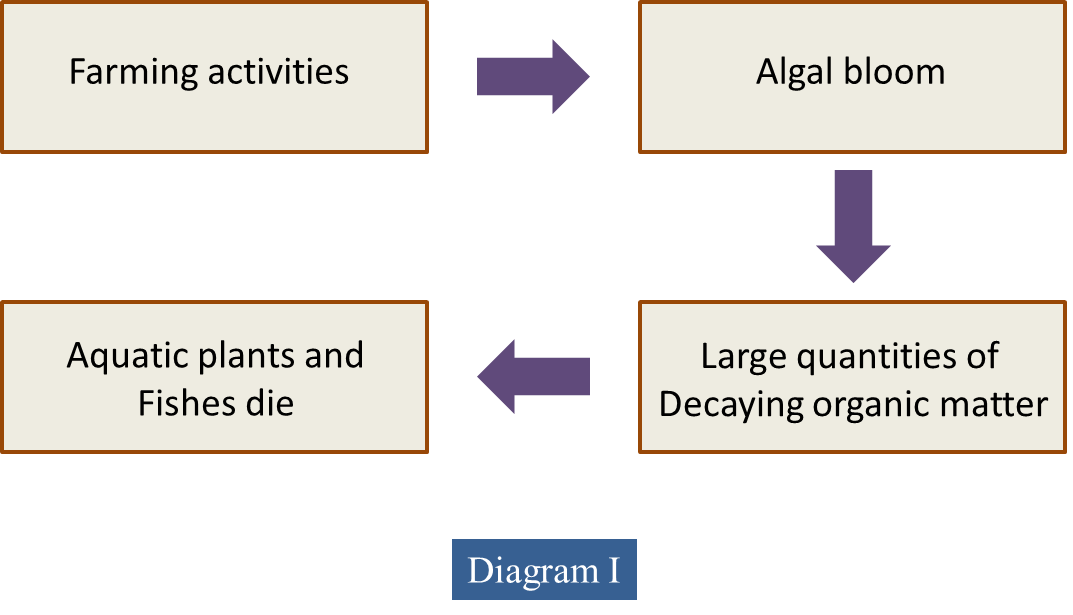
Explain how the farming activities cause the death of the aquatic plants and the fishes in the pond.
[adinserter block="3"]
(b) Diagram II shows a new industrial area situated near a residential area.
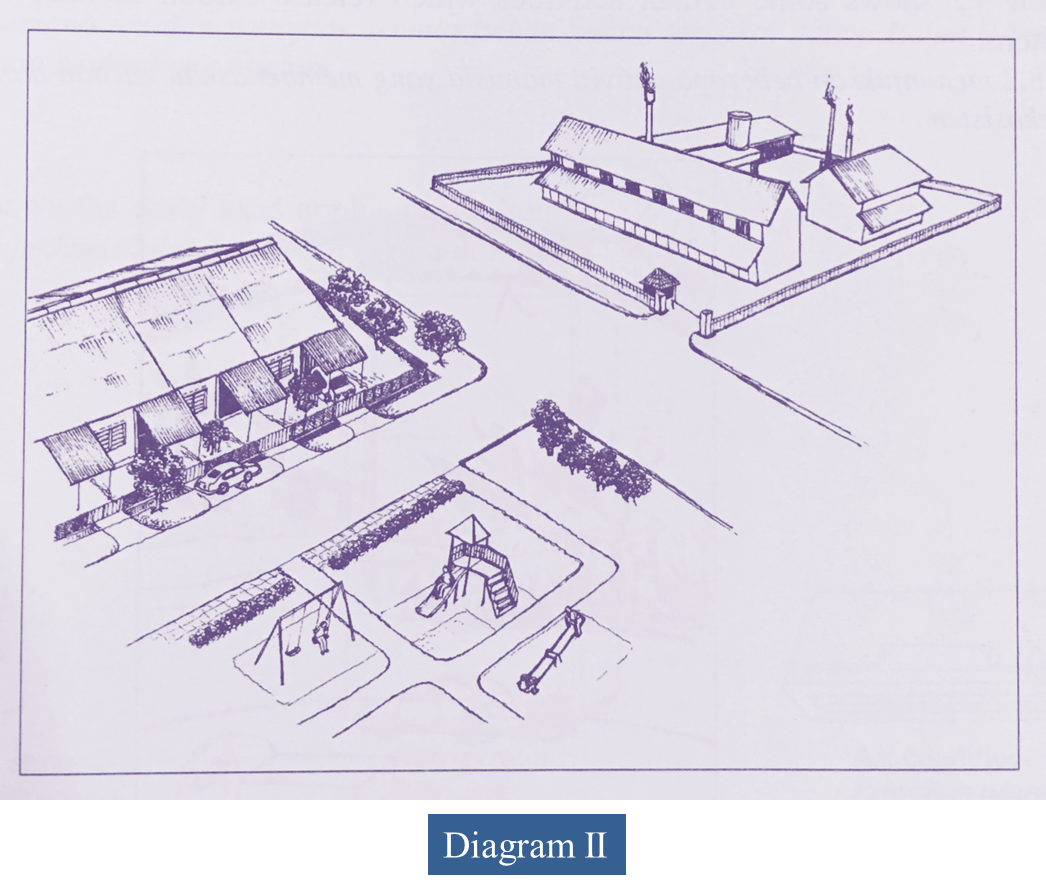
Discuss the good and the bad effects caused by the industrial activities on human and environment in years to come.
[adinserter block="3"]
Answer:
(a)
- Farmers use fertilisers that usually contain nitrates and phosphates.
- Fertilisers which contain nitrates/ phosphates may leach into the pond when it rains.
- Algae in the lake grow faster.
- They may grow so much that they completely cover the water.
- Black out the light for plants growing beneath them.
- Photosynthesis rate is reduced.
- Dissolved oxygen also reduced.
- Plants on the top of the water and beneath the water eventually die.
- Their remains are a good source of food for bacteria.
- Bacteria decomposed the dead plant rapidly.
- The large population of bacteria respires, using up oxygen, so there is very little oxygen left for other living organisms.
- BOD increased.
- Water population increases.
- Those aquatic plants and fish which need oxygen die.
[adinserter block="3"]
(b)
Advantages:
- More job opportunities
- More economic activities and development projects
- Attract tourists
- Improve infrastructure
Disadvantages:
- Can cause respiratory problems/ asthma/ bronchitis/ irritates the eye
- Crime rate increases
Environment:
- Industries emit poisonous gases such as sulphur dioxide/ oxides of nitrogen/ smoke/ fine solid particles
- Contribute to air pollution
- Oxides of nitrogen and Sulphur dioxide dissolve in rain water to form acid rain.
- Makes the soil acidic and unsuitable for the cultivation of crops
- Smoke and haze reduce light intensity reaching stomata and cause the rate of photosynthesis to decrease
- Which subsequently reduces crop yield
- Carbon dioxide leads to the greenhouse effect, resulting in an increase in the atmospheric temperature
- Cause the extinction of organisms
(a) Diagram I shows the effects of farming activities near a pond.

Explain how the farming activities cause the death of the aquatic plants and the fishes in the pond.
[adinserter block="3"]
(b) Diagram II shows a new industrial area situated near a residential area.

Discuss the good and the bad effects caused by the industrial activities on human and environment in years to come.
[adinserter block="3"]
Answer:
(a)
- Farmers use fertilisers that usually contain nitrates and phosphates.
- Fertilisers which contain nitrates/ phosphates may leach into the pond when it rains.
- Algae in the lake grow faster.
- They may grow so much that they completely cover the water.
- Black out the light for plants growing beneath them.
- Photosynthesis rate is reduced.
- Dissolved oxygen also reduced.
- Plants on the top of the water and beneath the water eventually die.
- Their remains are a good source of food for bacteria.
- Bacteria decomposed the dead plant rapidly.
- The large population of bacteria respires, using up oxygen, so there is very little oxygen left for other living organisms.
- BOD increased.
- Water population increases.
- Those aquatic plants and fish which need oxygen die.
[adinserter block="3"]
(b)
Advantages:
- More job opportunities
- More economic activities and development projects
- Attract tourists
- Improve infrastructure
Disadvantages:
- Can cause respiratory problems/ asthma/ bronchitis/ irritates the eye
- Crime rate increases
Environment:
- Industries emit poisonous gases such as sulphur dioxide/ oxides of nitrogen/ smoke/ fine solid particles
- Contribute to air pollution
- Oxides of nitrogen and Sulphur dioxide dissolve in rain water to form acid rain.
- Makes the soil acidic and unsuitable for the cultivation of crops
- Smoke and haze reduce light intensity reaching stomata and cause the rate of photosynthesis to decrease
- Which subsequently reduces crop yield
- Carbon dioxide leads to the greenhouse effect, resulting in an increase in the atmospheric temperature
- Cause the extinction of organisms
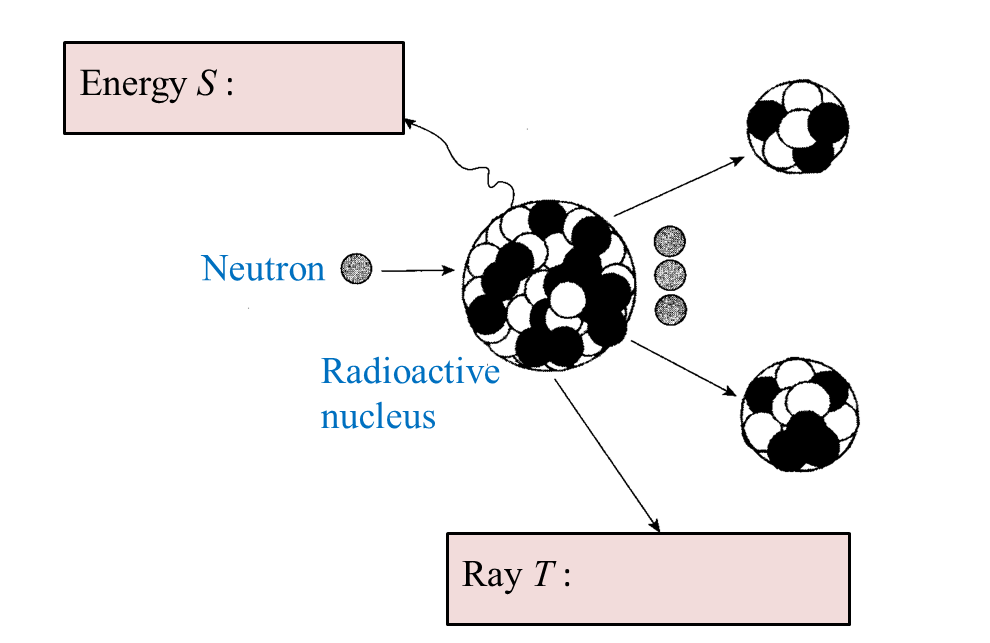
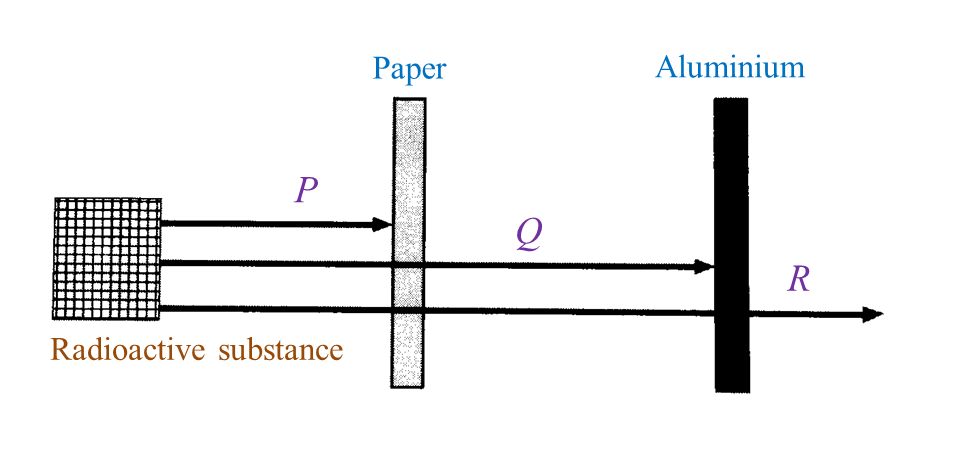
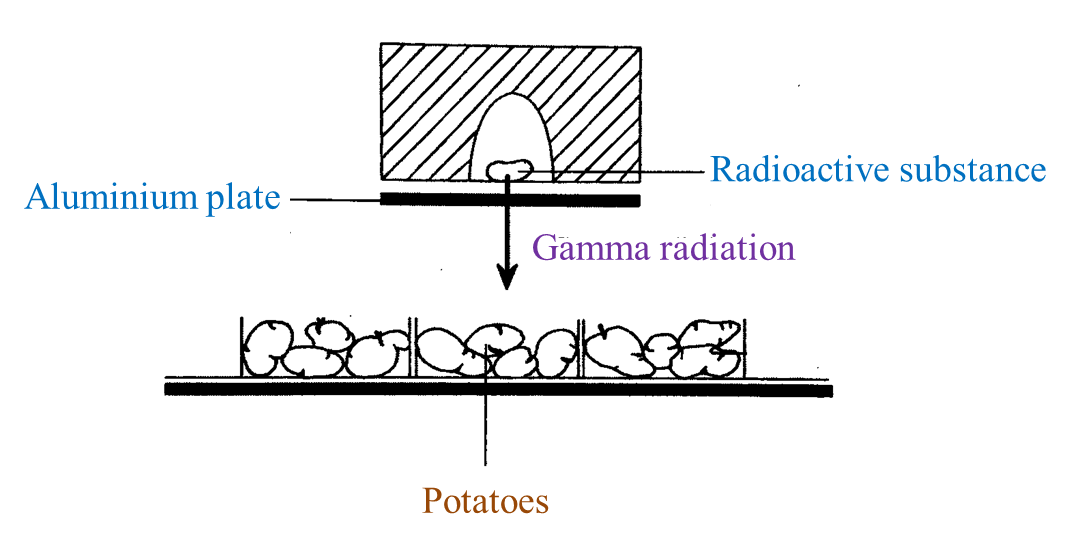
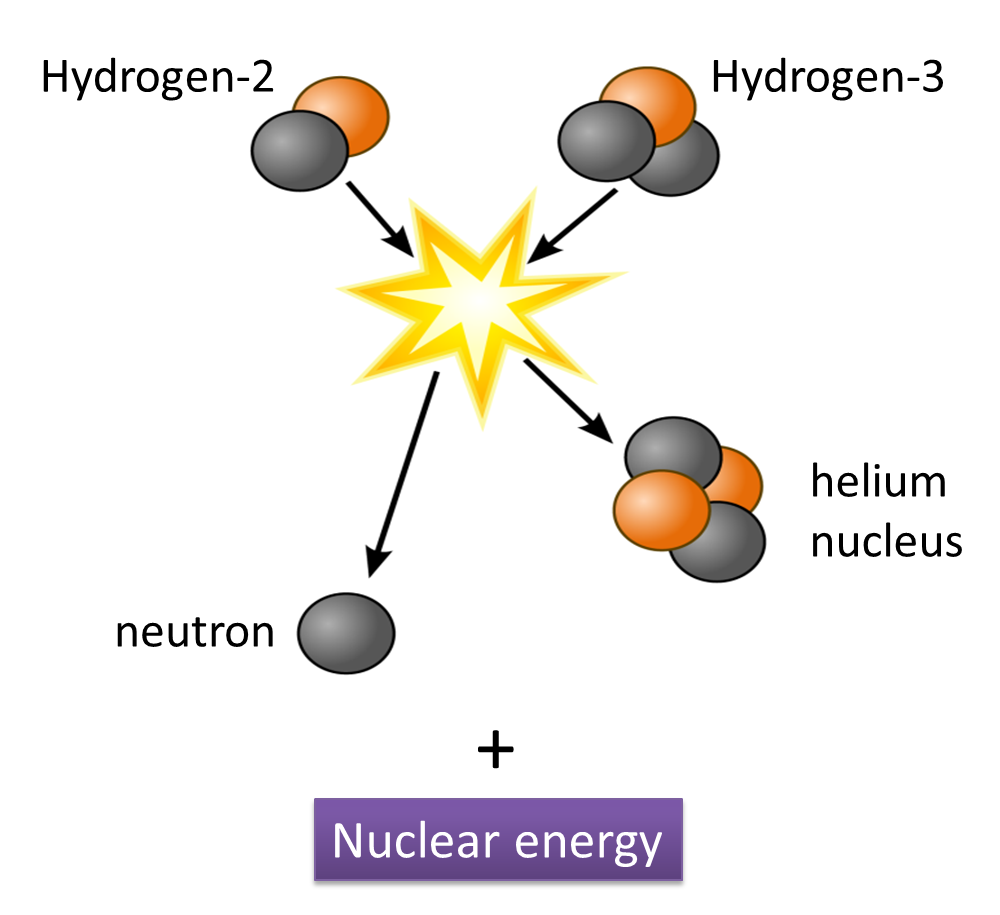
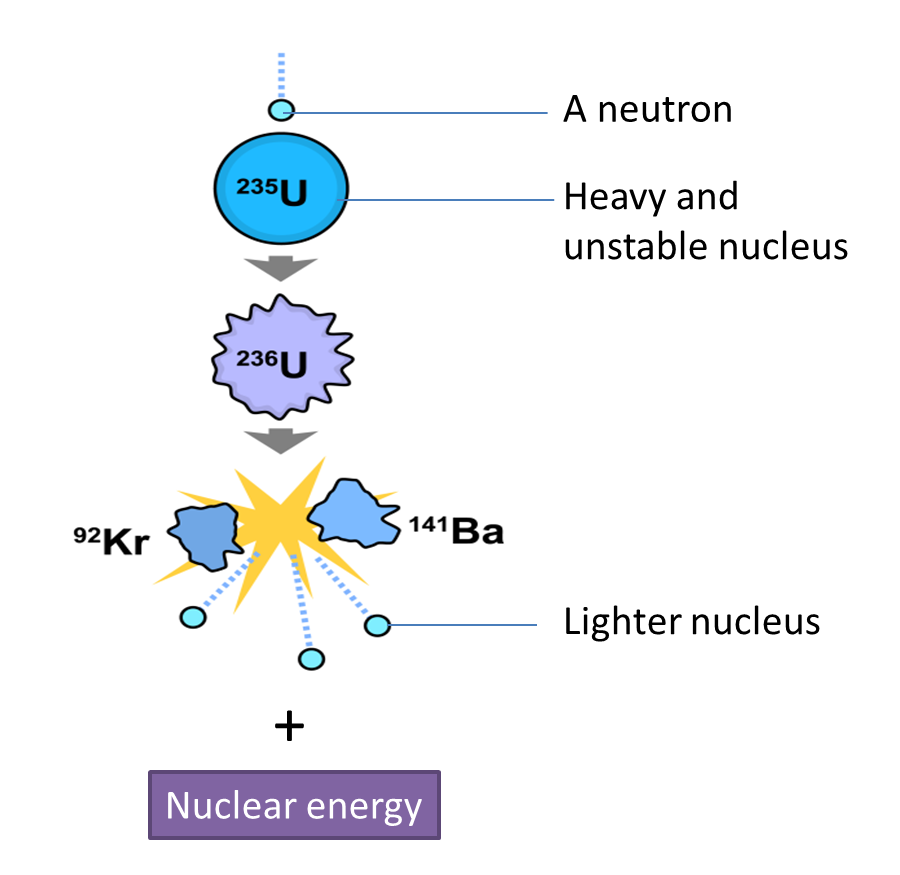
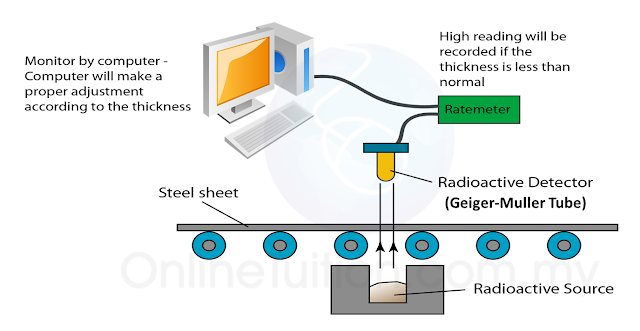
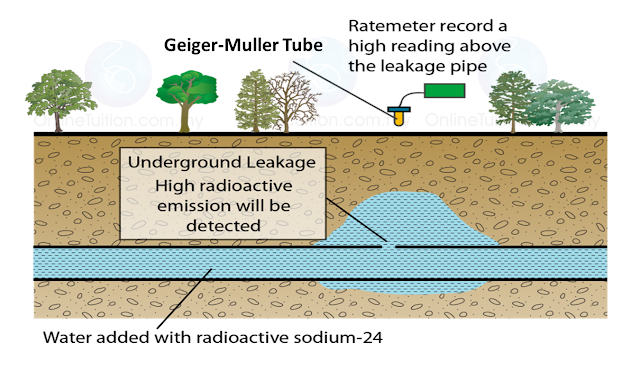
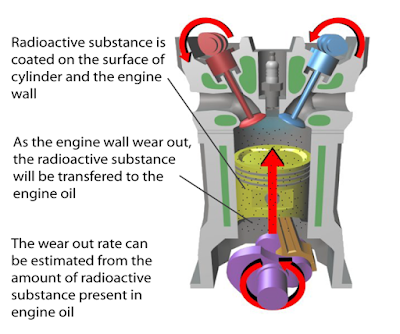
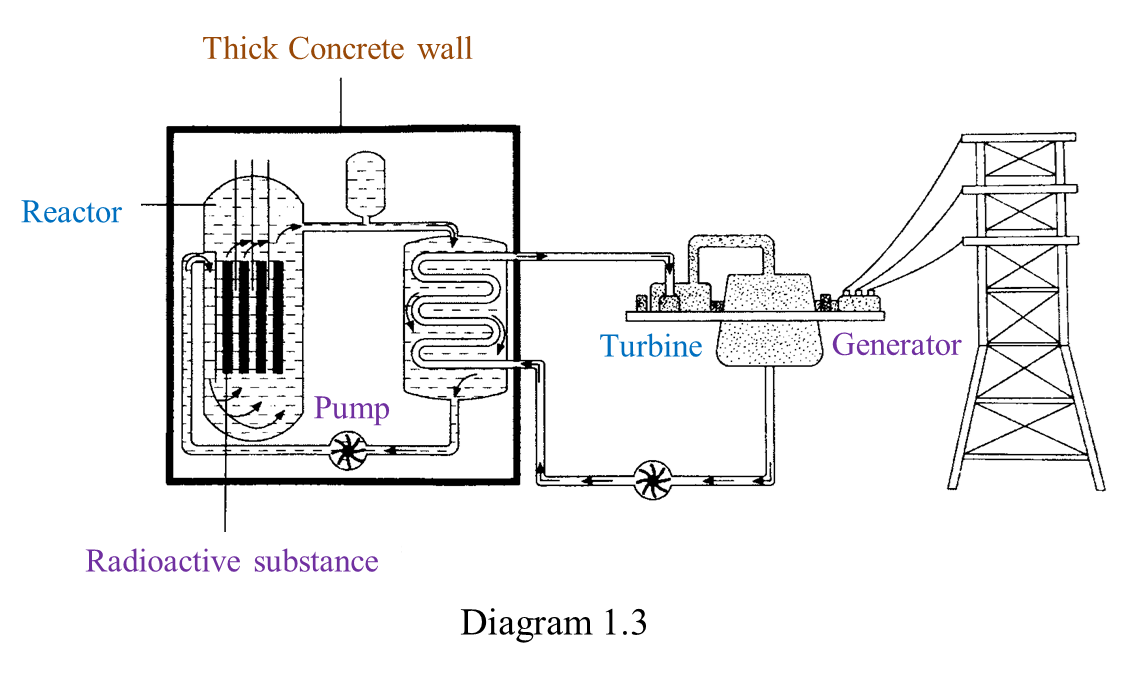 (i) Name the radioactive substances used in Diagram 1.3. [1 mark]
(i) Name the radioactive substances used in Diagram 1.3. [1 mark]