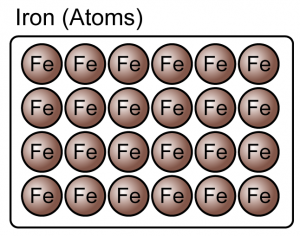
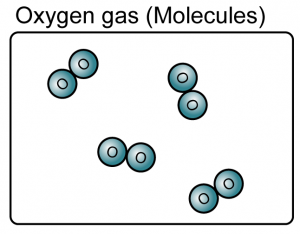
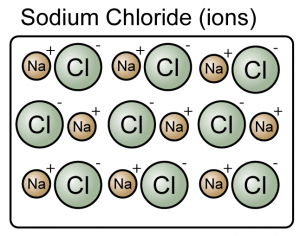
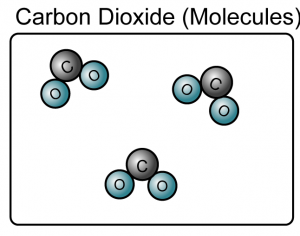
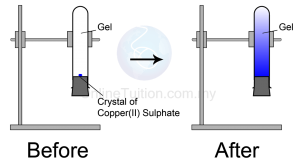
Matter can be divided into elements and compounds.


Elements
- An element is a substance that consists of only one type of atom.
- Element can be either atoms or molecules.
(Both the iron and oxygen are element because they consist of only one type of atoms)
Compounds
- A compound is a substance composed of molecules made up of atoms of two or more elements.
- A compound is made up of either molecules or ions.
(Both the sodium chloride and carbon dioxide are compound because they consist of more than one type of atoms)