- A p-type semiconductor can be produced by doped some trivalent atoms into a semiconductor.
- Trivalent atom is atom has only three valence electrons. Examples include aluminum, boron, and gallium.
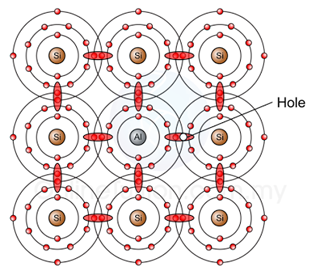
- Figure above shows an aluminium atom (which is trivalent ) in the center, surrounded by four silicon atoms.
- We can see that, the trivalent atom form 4 covalent bonds with the silicon atoms around. Since the trivalent atom has only three valence electrons and each neighbour shares one electron, only seven electrons are in the valence orbit..
- This means a hole exists in the valence orbit of each trivalent atom. A trivalent tom is also called an acceptor atom because each hole it contributes can accept a free electron.
- The more trivalent impurity that is added, the more holes in the semiconductor, and hence the greater the conductivity of the semiconductor.
- Some free electrons will also formed in the semiconductor when some electrons are promoted to shell with higher energy level.
- The holes outnumber the free electrons, hence they are called the majority carrier and the free electrons are called the minority carriers.
- Since the positive charge carrier (the holes) outnumber the negative charge carrier (the free electrons), the semiconductor is called a p-type semiconductor, where the p stands for positive.
| p-type semiconductor | n-type semiconductor | |
| Doping Material | Trivalent: aluminum, boron, and gallium |
Pentavalent: antimony, and phosphorus |
| Role of doping material | Atom receiver | Atom donor |
| Majority Charge Carrier | Holes | Free electrons |
| Minority Charge Carrier | Free electrons | Holes |