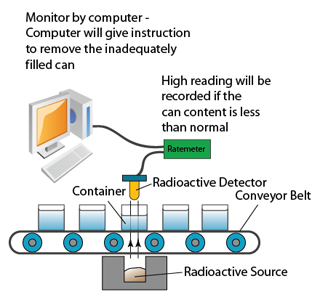
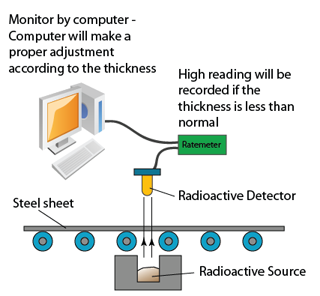
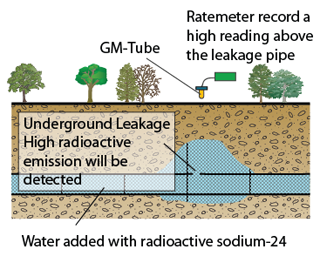
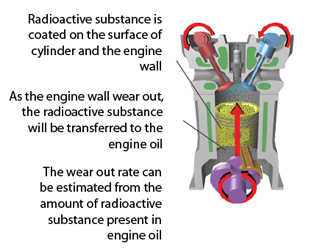
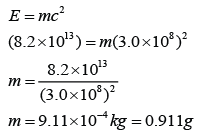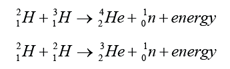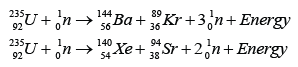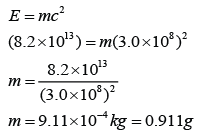Nuclear Reaction
- In a nuclear reaction, the mass of the parent particles will become less (know as mass defect). The defected mass is then converted into energy called the nuclear energy.
- In short, nuclear energy is the energy released owing to the defect of mass in a nuclear reaction.
- There are 2 types of nuclear reaction
- nuclear fission
- nuclear fusion
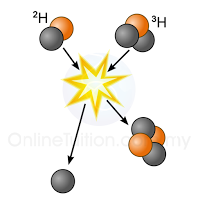
- Nuclear fission is the process of splitting nucleus into 2 smaller nuclei whereas nuclear fusion is the process which 2 small nuclei combine to form a larger nucleus.
Nuclear Fission
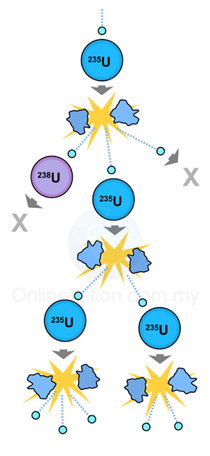
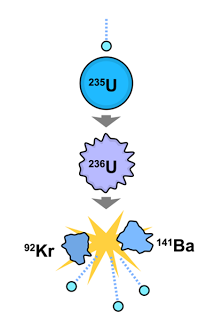
- Nuclear fission is a process involving the splitting of a heavy nucleus into two nuclei of roughly equal mass and shooting out several neutrons at the same time.
- Nuclear fission seldom occurs spontaneously. Usually, it occurs when the heavy nucleus is bombarded by a neutron.
- Fission reaction resulting from neutron absorption is called induced fission. Nuclei that undergo fission without initial neutron absorption are undergoing spontaneous fission.
- Two typical examples of fission reactions:
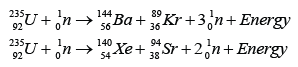
Example 1
In a nuclear reaction, the mass difference in the reaction is 1.5 x 10-8kg. Find the heat released in this reaction. [Speed of light = 3.0 x 108 ms-1]
Answer:
Mass defect, m = 1.5 x 10-8kg
Heat released,
E = mc²
E = (1.5 x 10-8)(3 x 108)
E = 1.35 x 109 J
Example 2
A nuclear explosion released 8.2 x 1013 J of energy. What is the mass defect of uranium-235 in this reaction?
[Speed of light = 3.0 x 108 ms-1]
Answer