Question 1:
Diagram I shows the organelles involved during the synthesis and secretion of an enzyme in an animal cell.
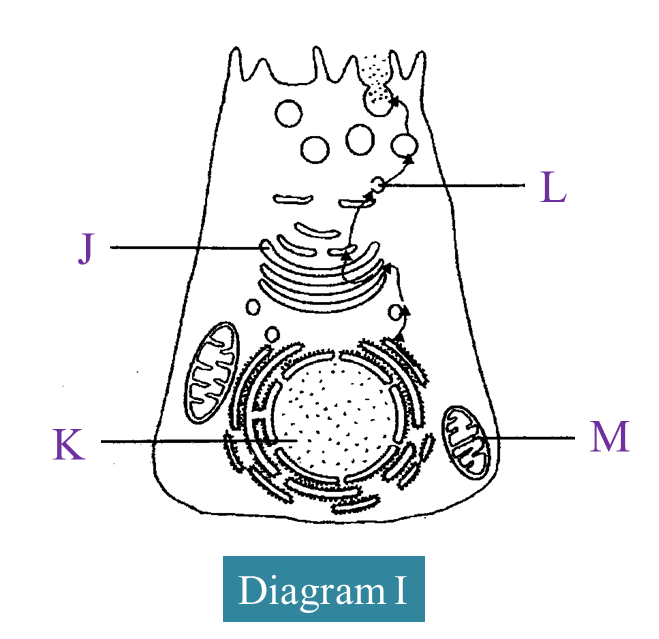
(a) Name the organelles labelled J and L.
(b)(i) State the function of organelle M.
(b)(ii) Explain the role of organelle K in the synthesis of the enzyme.
(c)
Explain how enzymes act in:
(i) helping to cook meat.
(ii) extracting agar from seaweeds.
(d) Diagram II shows the structure of an enzyme and three substrates W, X and Y.
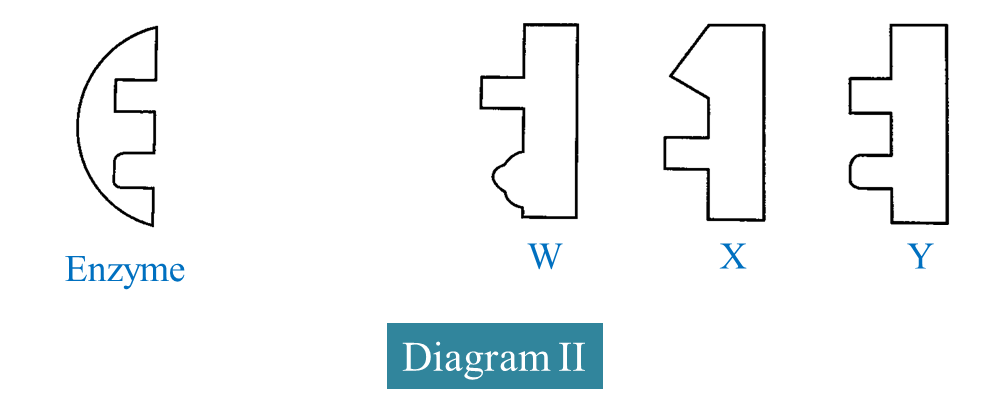
Based on Diagram II, complete the schematic diagram below to show the mechanism of enzyme action on a suitable substrate.
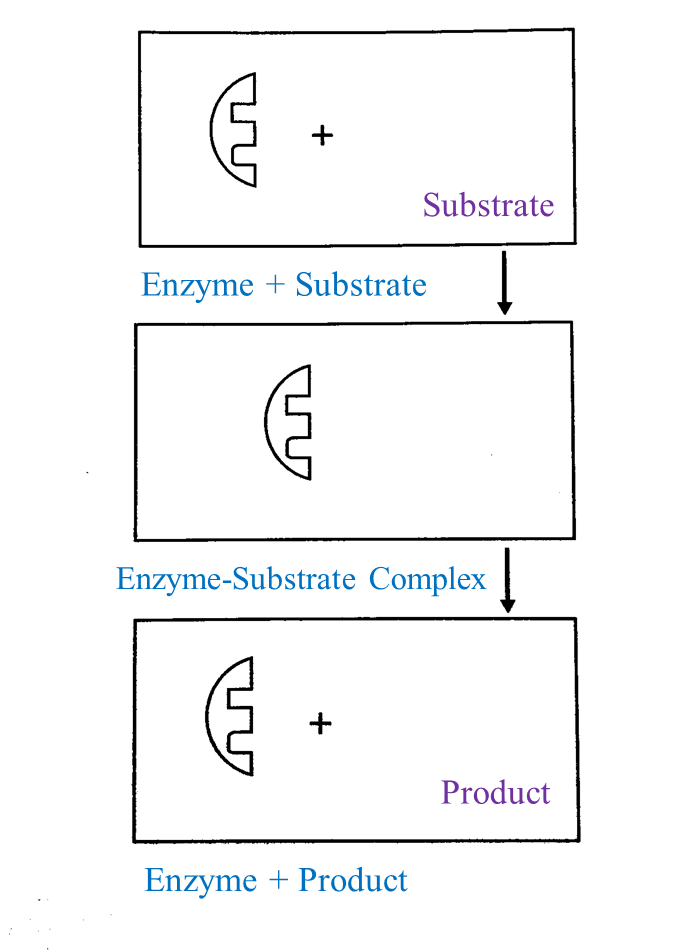
Answer:
(a)
J: Golgi apparatus
L: Secretory vesicles
(b)(i)
Produces energy
(b)(ii)
K stores genetic information in the DNA and this information is transferred to the RNA which then carries it out to the cytoplasm.
(c)(i)
Protease enzyme softens the meat.
(c)(ii)
Cellulase breaks down cell walls of seaweed and frees agar contained in it.
(d)
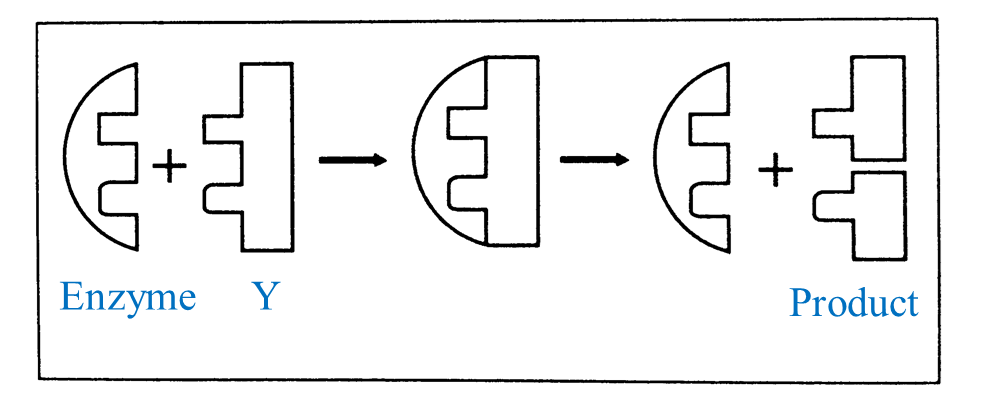
Diagram I shows the organelles involved during the synthesis and secretion of an enzyme in an animal cell.

(a) Name the organelles labelled J and L.
(b)(i) State the function of organelle M.
(b)(ii) Explain the role of organelle K in the synthesis of the enzyme.
(c)
| Enzymes are widely used in our daily life and industries. |
(i) helping to cook meat.
(ii) extracting agar from seaweeds.
(d) Diagram II shows the structure of an enzyme and three substrates W, X and Y.

Based on Diagram II, complete the schematic diagram below to show the mechanism of enzyme action on a suitable substrate.

Answer:
(a)
J: Golgi apparatus
L: Secretory vesicles
(b)(i)
Produces energy
(b)(ii)
K stores genetic information in the DNA and this information is transferred to the RNA which then carries it out to the cytoplasm.
(c)(i)
Protease enzyme softens the meat.
(c)(ii)
Cellulase breaks down cell walls of seaweed and frees agar contained in it.
(d)