Question 1:
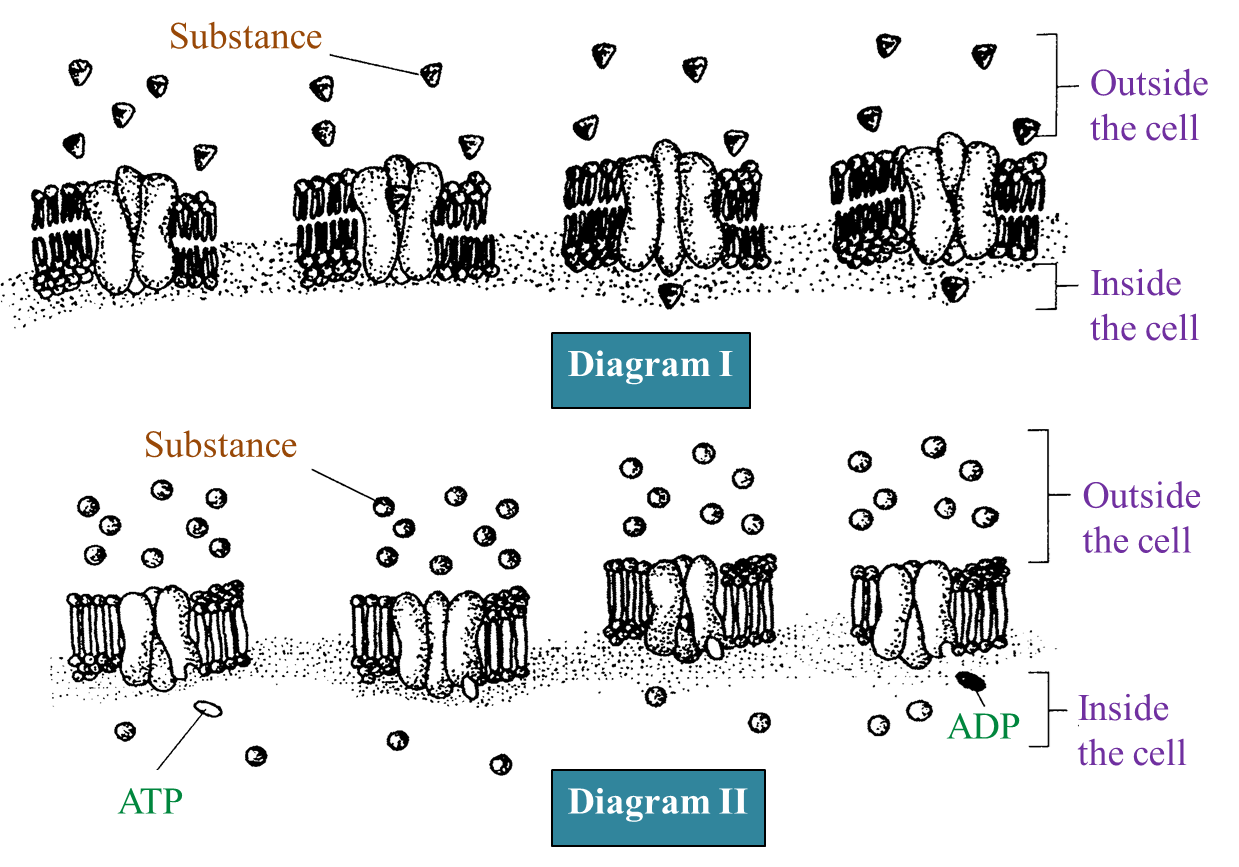
Diagram I and Diagram II show the movement of substances P and Q across the plasma membrane respectively. The movement of P needs energy but the movement of Q does not.
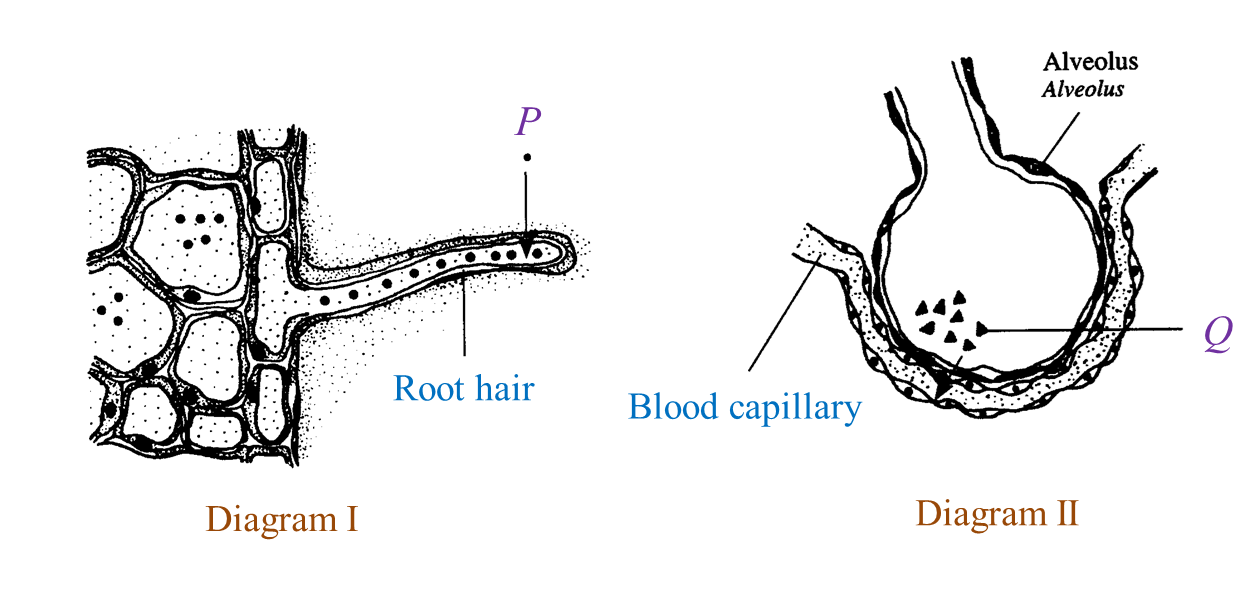 (a)
(a) Name the processes involved in the movement of P and Q.
(2 marks)
(b)(i) Name
one example of P and Q.
(2 marks)(b)(ii) Describe the movement of Q shown in Diagram II.
(2 marks)
(c) Diagram III shows the condition of a plant cell before and after being immersed in a type of solution.
(3 marks)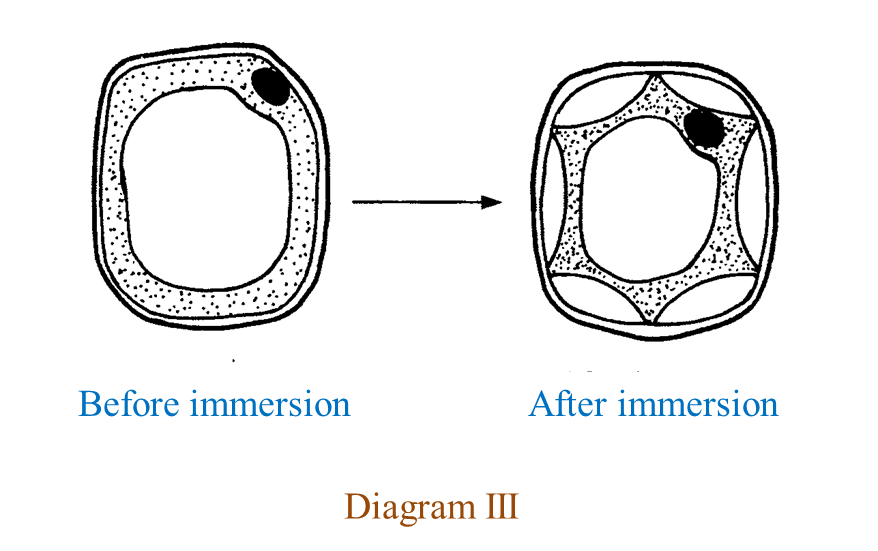
Explain the condition of the cell after being immersed in the solution.
(d) A housewife makes mango pickles by immersing mango slices in a concentrated sugar solution.
State
one advantage and
two disadvantages of the method used, compared to storing fresh mangoes.
(3 marks)
Answer:
(a)
P: Active transport
Q: Simple diffusion
(b)(i)
P: Nitrate
Q: Oxygen
(b)(ii)
The partial pressure of Q is higher in the alveolus than in the blood capillary. As a result, Q diffuses into the blood capillary following the concentration gradient.
(c)
The plasma membrane is pulled away from the cell wall, the vacuole becomes small, as a result the cell becomes flaccid and plasmolysis occurs.
(d)
Advantage: It keeps food for longer period.
Disadvantages:
1. The sugar content of the food is too high.
2. Some of the nutrients such as vitamin C are lost.
Question 2:
Diagram I below shows a plant cell that has been immersed in 30% sucrose solution.
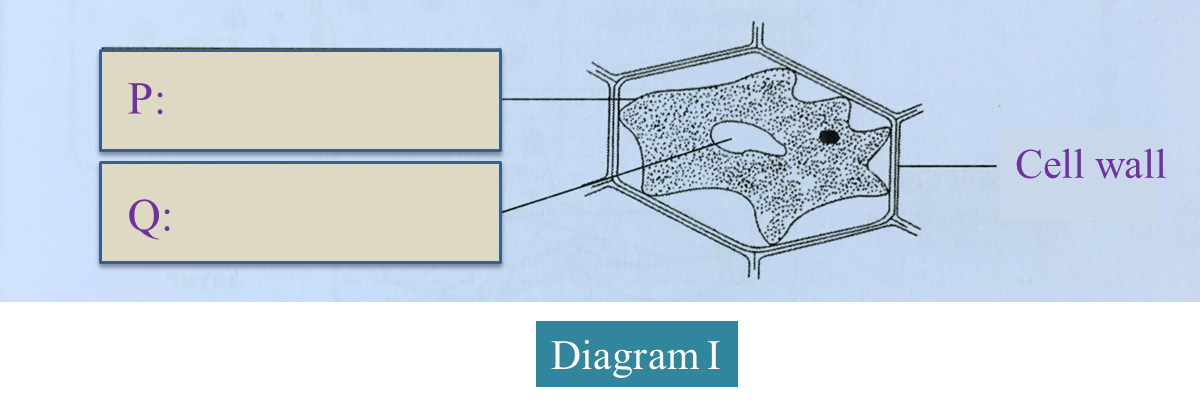
(a)(i) On the diagram, label P and Q.
(2 marks)(a)(ii) Name the solution which filled the space between the cell wall and P.
Explain how the solution filled the space.
(2 marks)
(b) The plant cell in the Diagram has undergone plasmolysis.
Explain how this happened.
(2 marks)
(c) Diagram II and III below show the condition of two plants which are added with fertilizer. The plant in Diagram III is added with excess fertilizer.
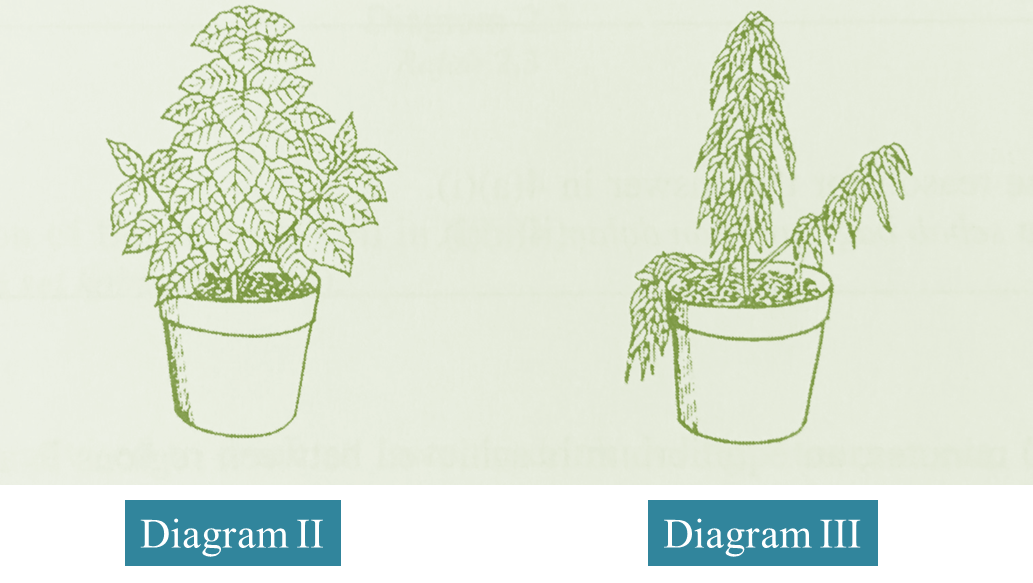
Explain the condition of the plant in Diagram III.
(3 marks)
(d) Diagram IV shows a method of preserving vegetables.
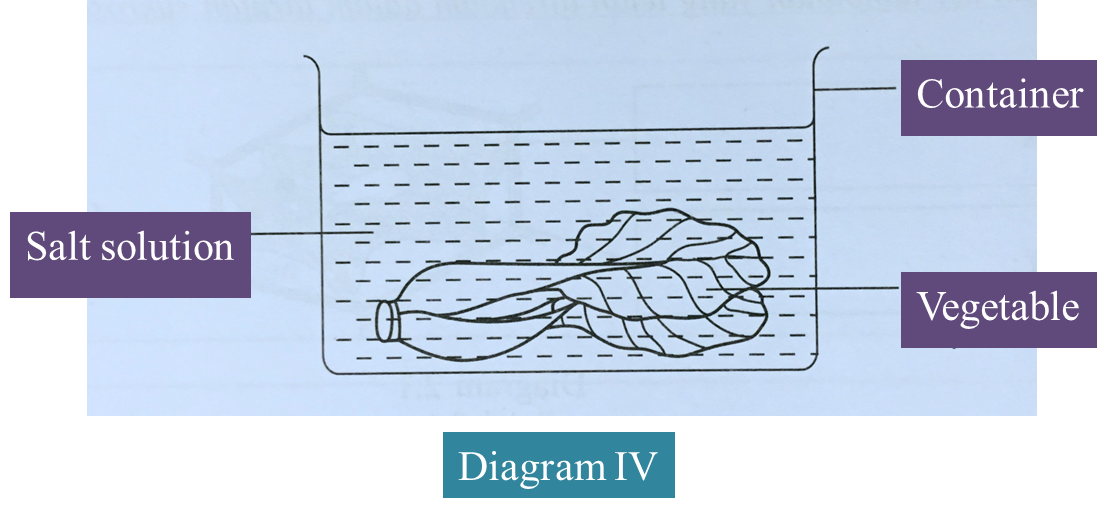
Explain the method used.
(3 marks)
Answer:
(a)(i)
P – Plasma membrane
Q – Vacuole
(a)(ii)
Solution: Sucrose solution
Explanation: Cell wall is permeable to allow sucrose solution to pass through and fill up the spaces.
(b)
When water molecules diffuse out of the large vacuole by osmosis, the plasma membrane will be pulled away from the cell wall. The cytoplasm shrinks due to osmosis.
(c)
Excess fertilizer will cause the soil water to be hypertonic towards the root hair cells. As a result, water from the root hair cells diffuses out to the soil by osmosis. The cells become plasmolysed and this leads to wilting.
(d)
Salting – Concentrated salt solution is used to soak vegetables, The hypertonic solution causes vegetable tissues to be dehydrated. Microorganisms lose water by osmosis and cannot live without water.
 (a) Name the processes involved in the movement of P and Q. (2 marks)
(a) Name the processes involved in the movement of P and Q. (2 marks)