Skip to content
Skip to main menu
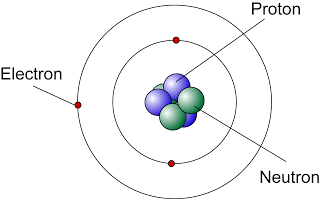
- You have learn in chemistry that in an atom, electrons move around a central core called the nucleus.
- The nucleus consists of protons and neutrons. It containing almost all the mass of the atom.
- The nucleus of an atom is very small compared to the size of the atom
- Protons and neutrons also known as nucleons.
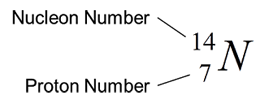
Nuclide Notation
- Proton number is defined as the number of protons in a nucleus.
- Nucleon number is defined as the total number of protons and neutrons in a nucleus. It is also known as mass number.
- A nuclide is a type of atom with a particular proton and nucleon number.
- A nuclide can be represented by a nuclide notation that shows the symbol of element, proton number and nucleon number.
- Figure below shows the nuclide notation of a nitrogen.