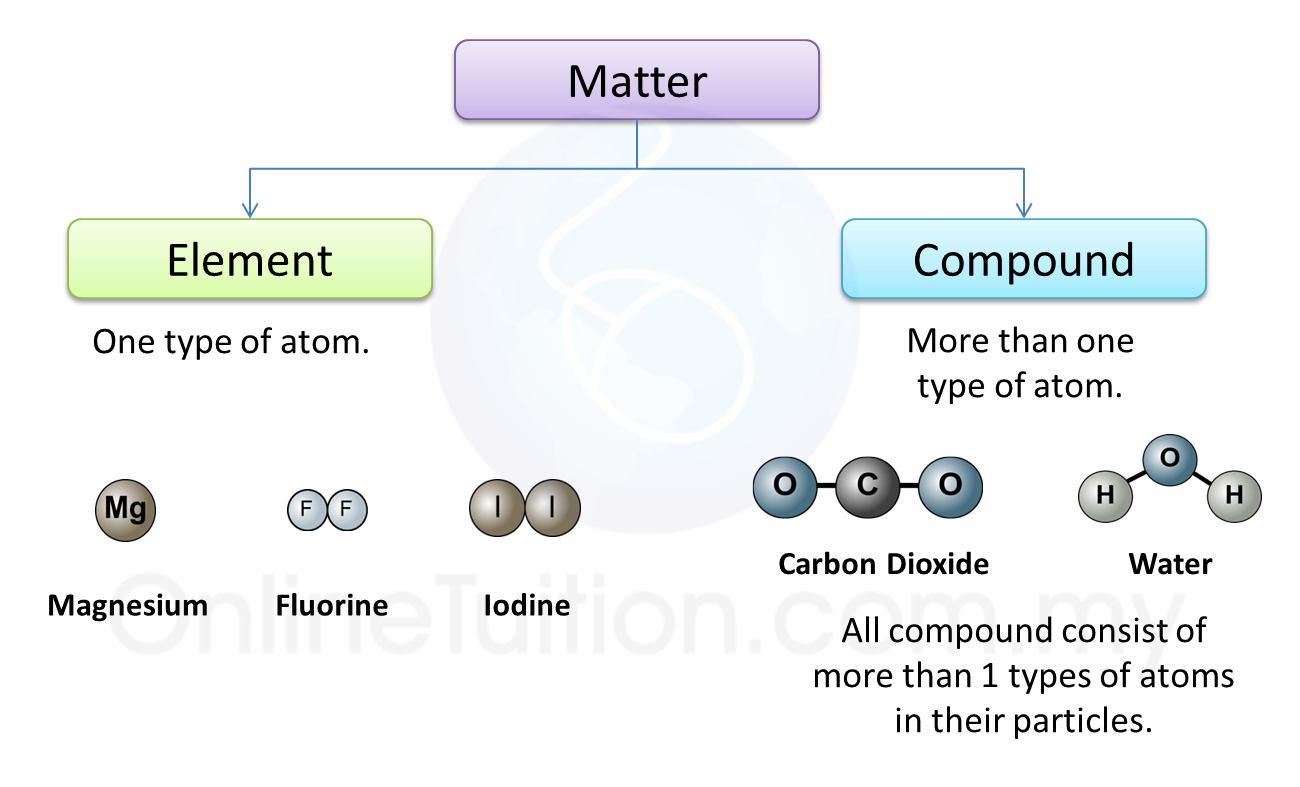
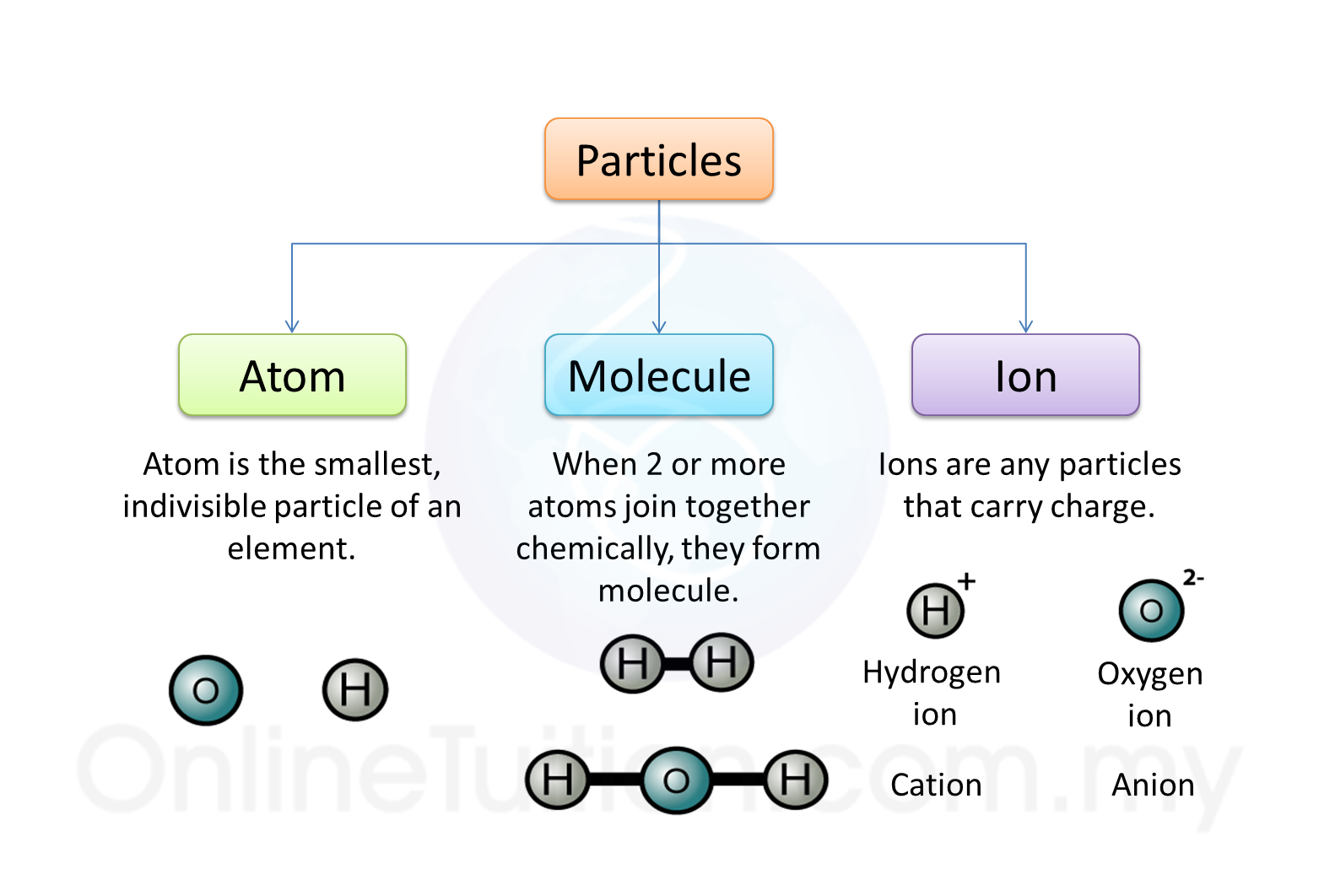
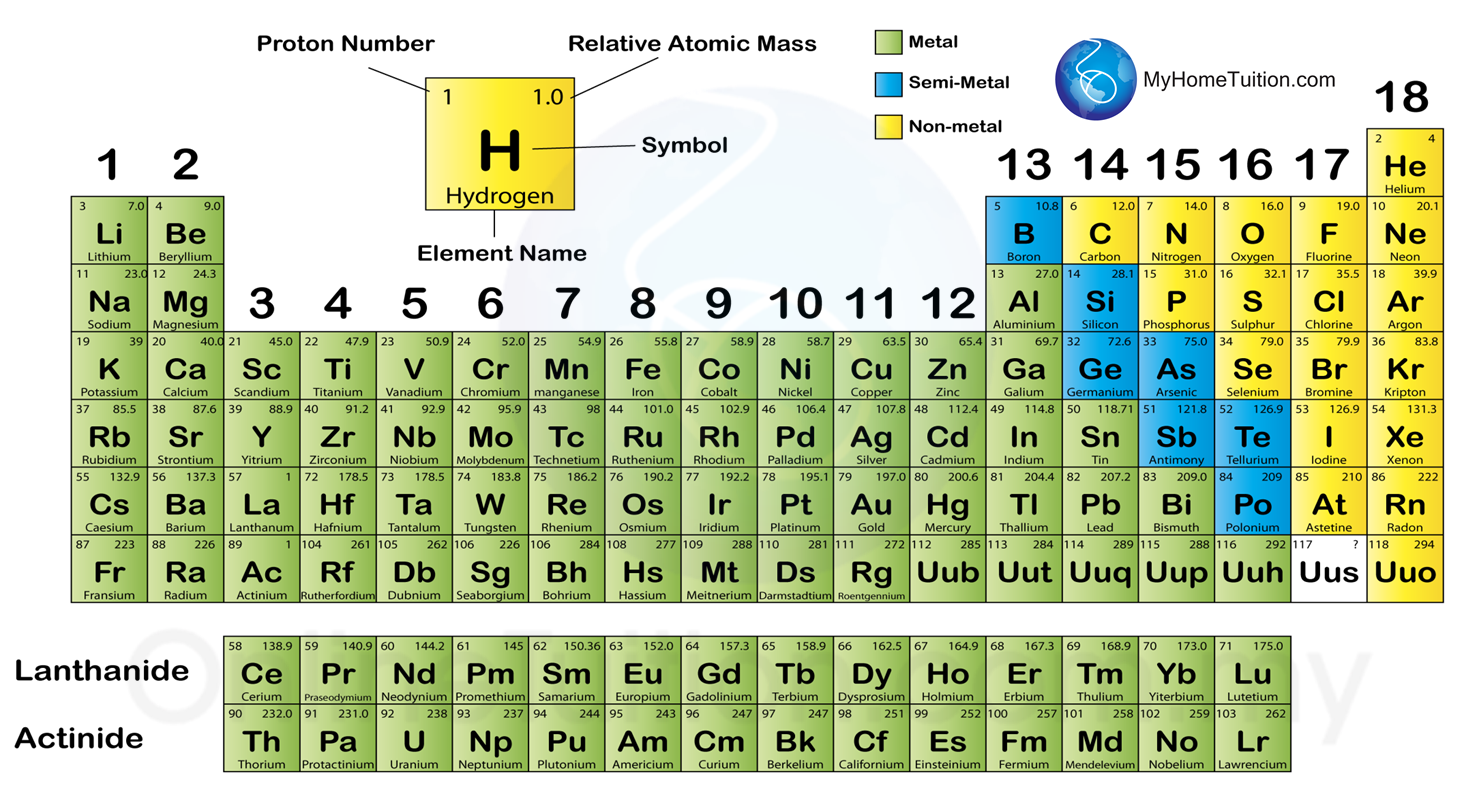
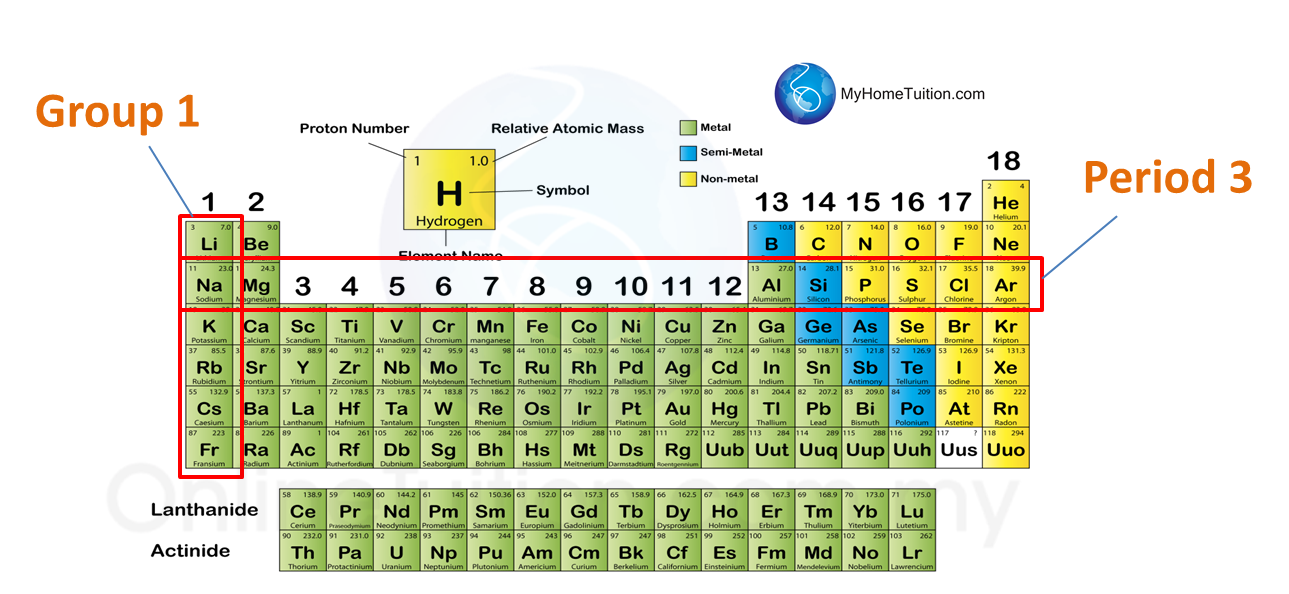
Question 1:
Diagram 1.1 and Diagram 1.2 show an experiment to study the electrical conductivity of lead(II) bromide.
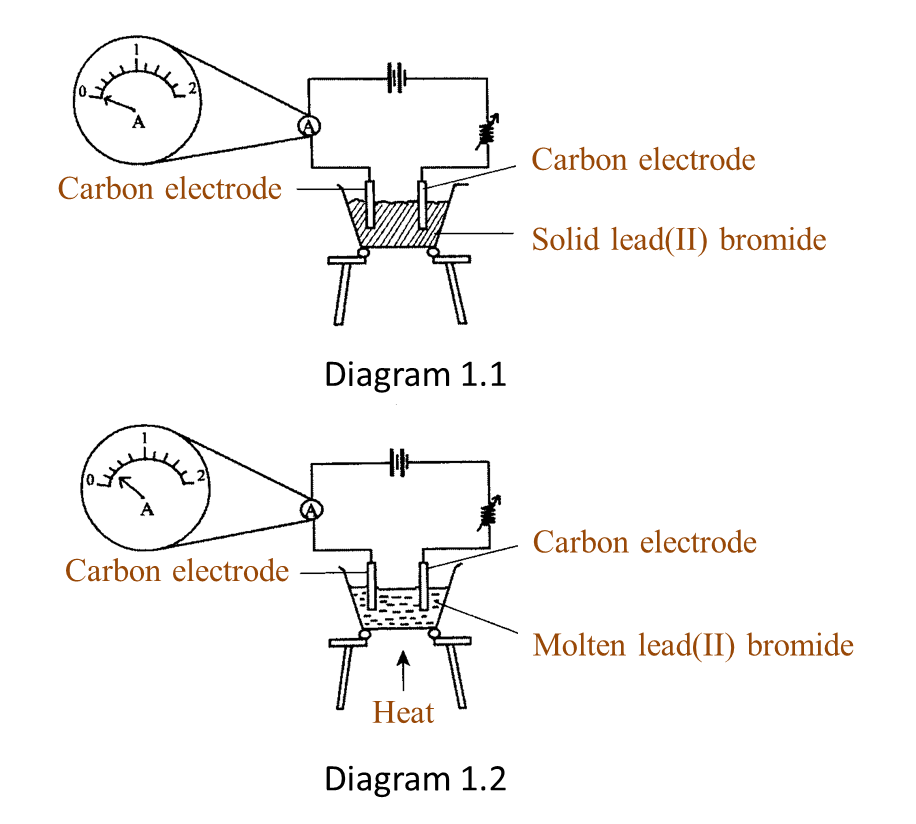
(a)(i) Based on Diagram 1.2, what is your observation on the needle of the ammeter? [1 mark]
(ii) What is the reading of the ammeter in Diagram 1.2? [1 mark]
(b) State the variables I this experiment.
(i) Manipulated variable:
(ii) Responding variable:
[2 marks]
(c) State one inference for this experiment. [1 mark]
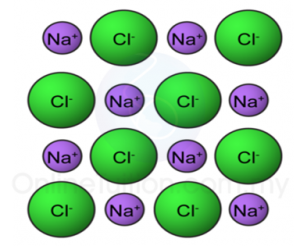
(d) Lead(II) bromide is an ionic compound.
State the operational definition for an ionic compound. [1 mark]
Answer:
(a)(i) The pointer of the ammeter is deflected.
(a)(ii) 0.4 A
(b)(i) The state of the lead(II) bromide or chemical compound
(b)(ii) The ammeter reading
(c) Lead(II) bromide in the molten state can conduct electricity.
(d) An ionic compound in the molten state will cause the pointer of the ammeter connected in a closed circuit to be deflected.
Diagram 1.1 and Diagram 1.2 show an experiment to study the electrical conductivity of lead(II) bromide.

(a)(i) Based on Diagram 1.2, what is your observation on the needle of the ammeter? [1 mark]
(ii) What is the reading of the ammeter in Diagram 1.2? [1 mark]
(b) State the variables I this experiment.
(i) Manipulated variable:
(ii) Responding variable:
[2 marks]
(c) State one inference for this experiment. [1 mark]
(d) Lead(II) bromide is an ionic compound.
State the operational definition for an ionic compound. [1 mark]
Answer:
(a)(i) The pointer of the ammeter is deflected.
(a)(ii) 0.4 A
(b)(i) The state of the lead(II) bromide or chemical compound
(b)(ii) The ammeter reading
(c) Lead(II) bromide in the molten state can conduct electricity.
(d) An ionic compound in the molten state will cause the pointer of the ammeter connected in a closed circuit to be deflected.
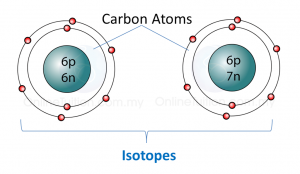
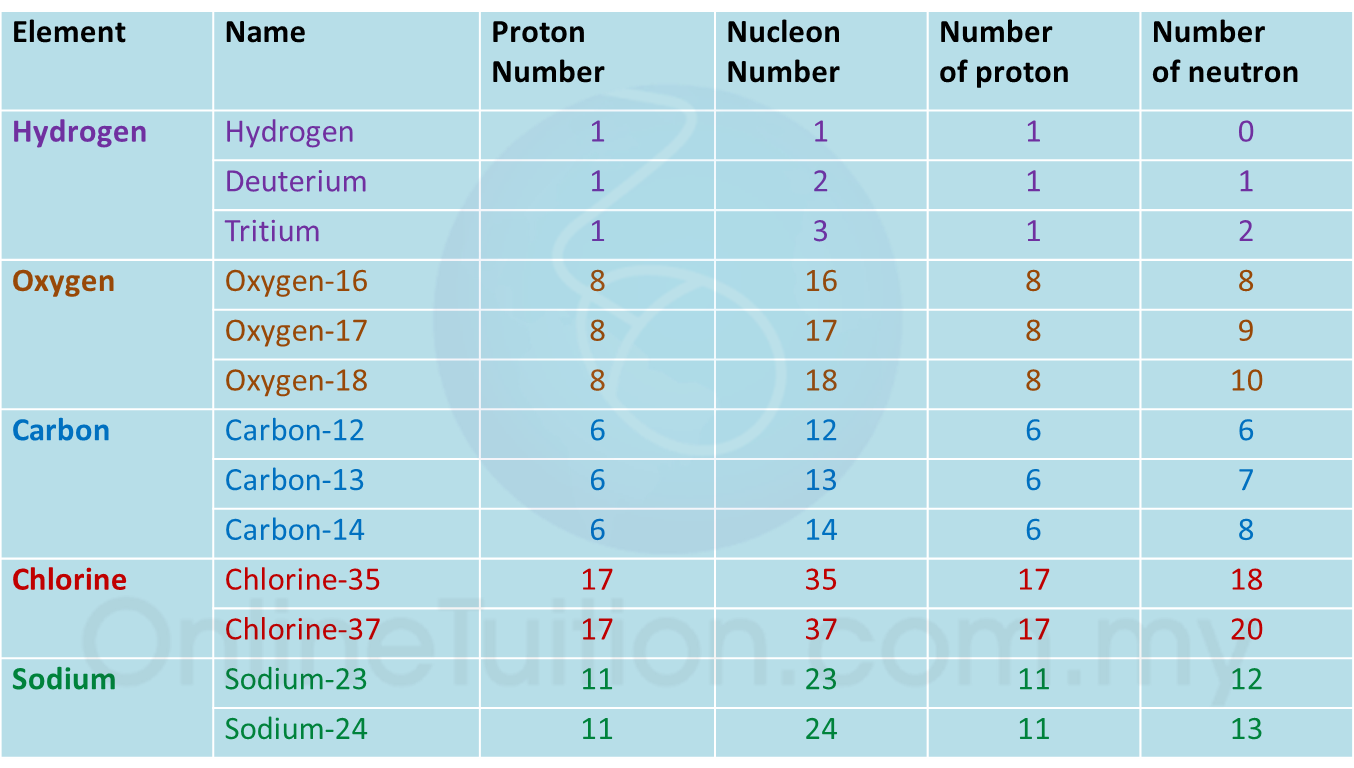
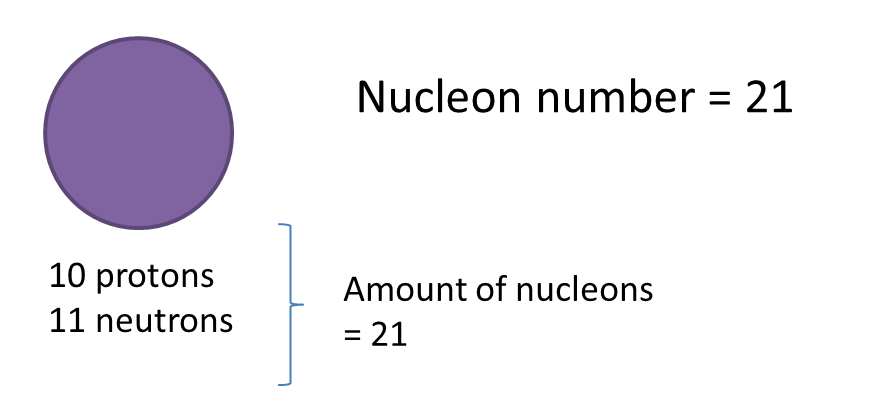
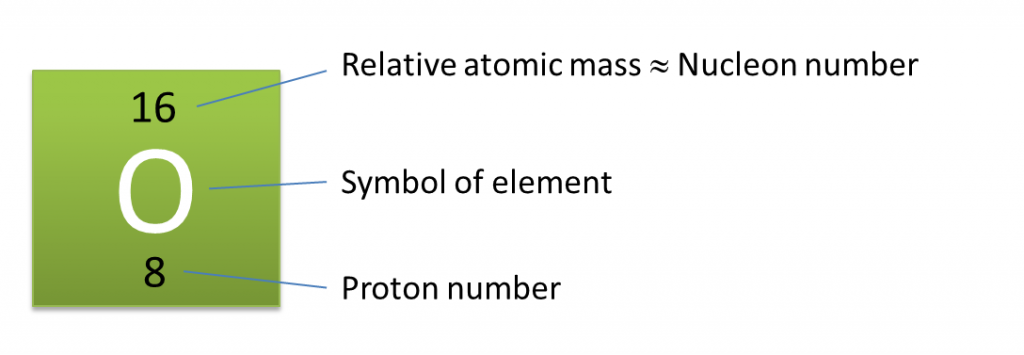
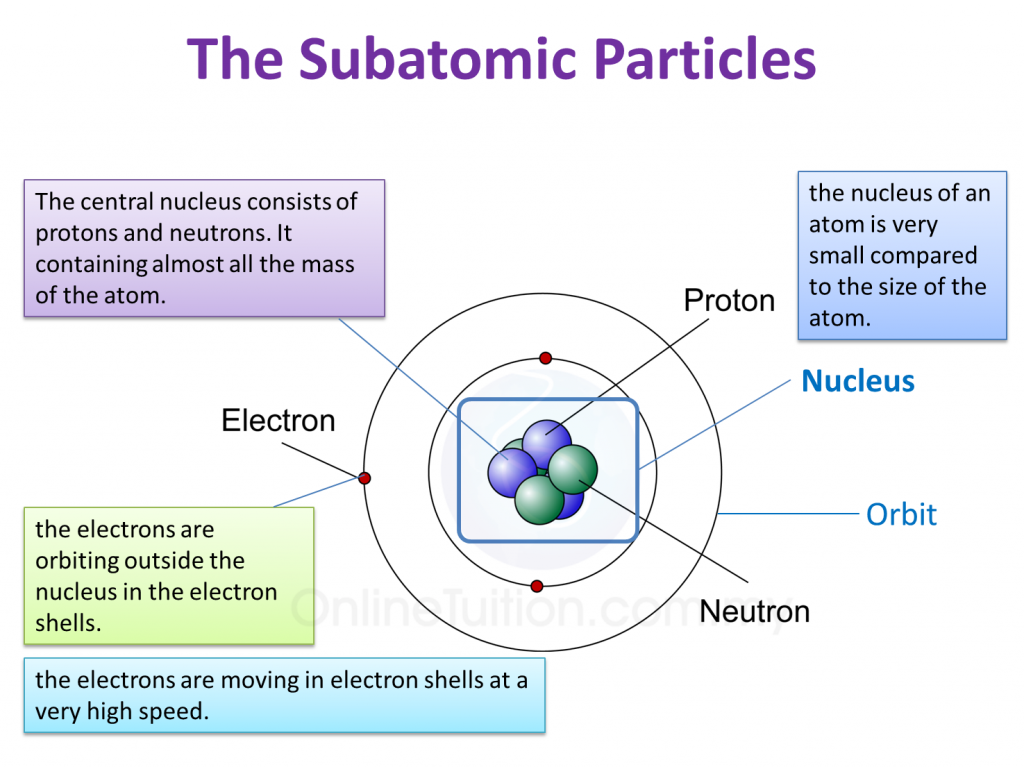
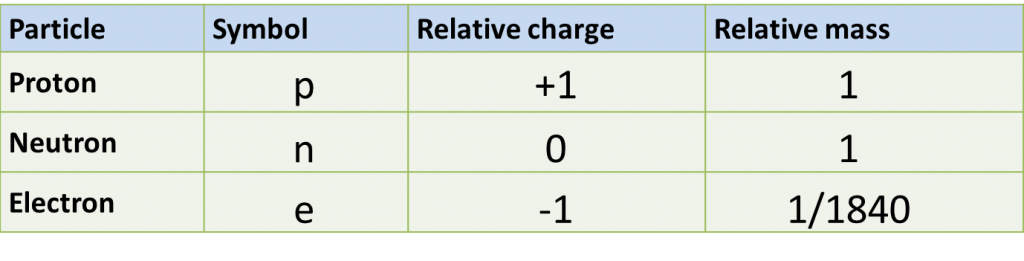
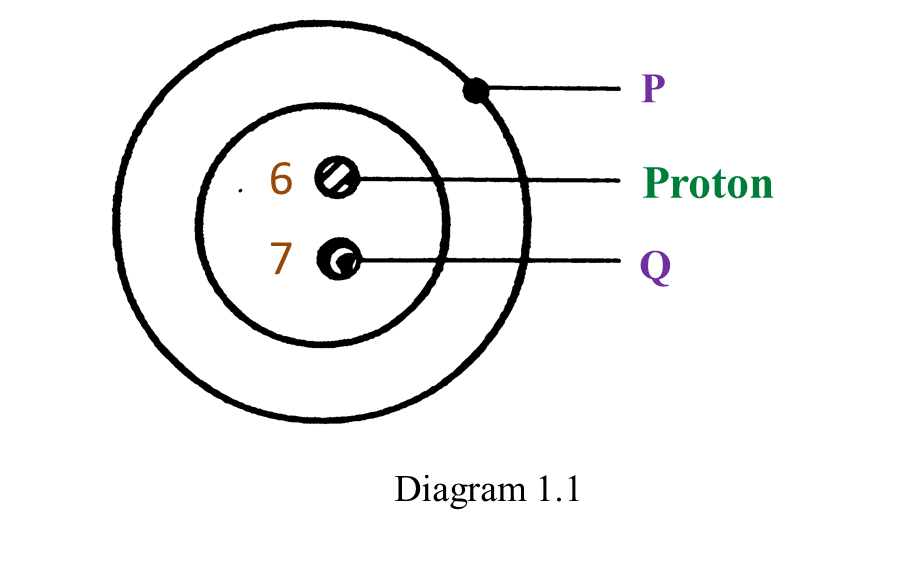 (a) P and Q are subatomic particles.
(a) P and Q are subatomic particles.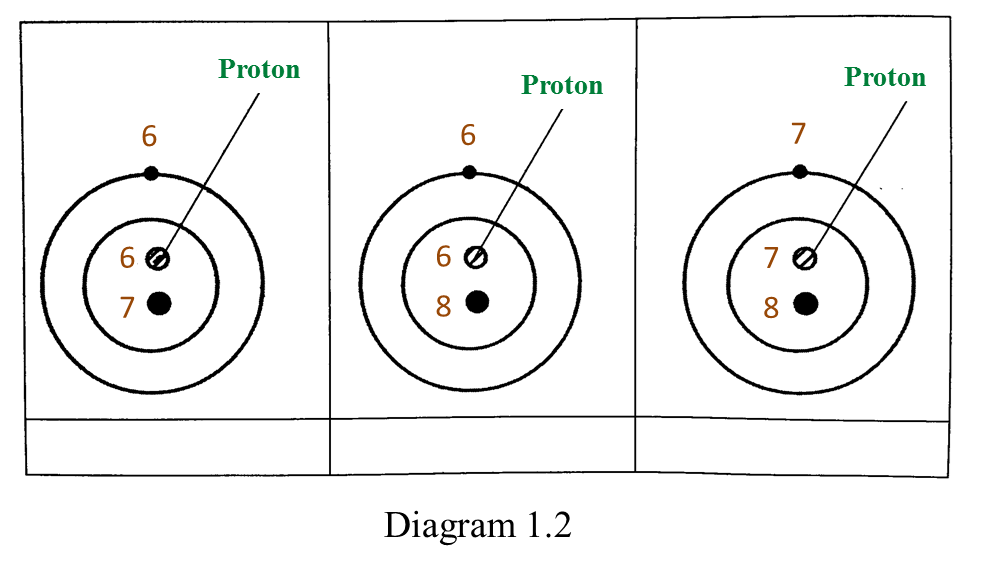
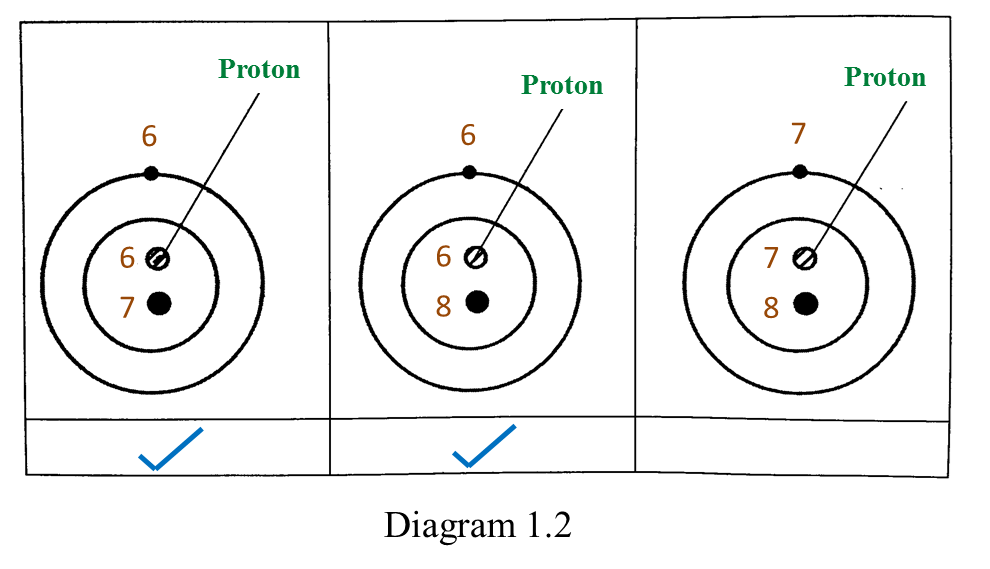
 Based on the Diagram,
Based on the Diagram,